Translate this page into:
Periampullary Adenocarcinoid: Diagnostic Dilemma
Address for correspondence: Dr. Rashmi Krishnappa, E-mail: rashmikrishnappa@yahoo.co.in
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The growth in periampullary region needs proper evaluation as they can arise from duodenum, pancreas and extra hepatic biliary tree. We have reported a case of adenocarcinoid of duodenum presenting as periampullary growth with obstructive jaundice. Histologically, tumor displayed biphasic pattern, i.e. tubuloglandular architecture with admixed sheets of neuroendocrine cells without pancreas and extra hepatic biliary tree involvement. The adenocarcinoids are of great diagnostic challenges for the pathologist interpreting endoscopic biopsy for the frequent misinterpretation as negative due to their submucosal location or as adenocarcinomas due to tubulo-glandular architecture.
Keywords
Adenocarcinoids
carcinoids
periampullary region
INTRODUCTION
The term adenocarcinoid was first coined by Warkel in 1978, to describe a group of uncommon low grade malignant tumor of appendix, with both morphological and histochemical evidence of glandular and neuroendocrine differentiation.[1]
Adenocarcinoids are also known as goblet cell carcinoid or composite carcinoids. They suggest the tumor with dual glandular and neuroendocrine differentiation. Though they are known to exist in various locations, they are very rare in periampullary region. They are commonly reported in appendix, colon, gall bladder[1] and stomach. In the periampullary region they are often associated with familial syndromes such as neurofibromatosis type 1, Zollinger Ellison syndrome etc.[2]
The adenocarcinoids are relatively indolent neoplasms which are not associated with carcinoid syndromes. They are often present with obstructive symptoms like mass effect.[3] It is important to distinguish these neoplasms from typical or classical carcinoids and also from adenocarcinoma.
The carcinoids at periampullary region are rare. Coexistence of well differentiated carcinoid with tubulo-glandular differentiation, i.e. adenocarcinoids is extremely rare entity.
CASE REPORT
A 52-year post menopausal lady came with complaints of pain in the abdomen and vomiting since one and half months, along with yellowish discoloration of eyes and pruritis. She also had low grade fever, black stools and loss of weight. On physical examination patient had icterus, pallor and tenderness of right hypochondrium.
The routine investigations showed deranged liver function tests with high direct bilirubin (21.4 mg/dl), aspartate amino transferace (AST) (134 IU/L), alanine amino transferace (ALT) (91 IU/L) and alkaline phosphatase (385 IU/L). She was negative for all viral serological markers of hepatitis and auto antibodies.
The ultrasound examination of the abdomen and pelvis showed small collection of fluid with internal septation and edematous jejunal loops in right perihepatorenal region. Pneumobilia with minimal intrahepatic biliary tract dilation noted.
The computed topography (CT) scan abdomen and pelvis showed an ill defined contrast enhancing intraluminal mass in distal second part of the duodenum with terminal CBD (common bile duct) encasing and causing significant luminal narrowing. Significant intra and extra hepatic dilation also noted. The pancreatic duct was normal. Radiological features favored a malignant growth.
Patient underwent endoscopy which showed intra luminal polypoidal mass measuring 2 cm in diameter at periampullary region. Biopsy from the same site was diagnosed as the non specific chronic duodenitis and was negative for malignancy. However, due to clinicoradiological suspicion of malignancy patient underwent Whipple's procedure.
Macroscopic examination of the resected specimen showed well defined single grey white submucosal mass in the duodenum adjacent to Ampulla of Vater measuring 2 × 1 cm [Figure 1]. The pancreas and CBD were uninvolved by the growth. Periampullary region showed 6 enlarged lymph nodes.
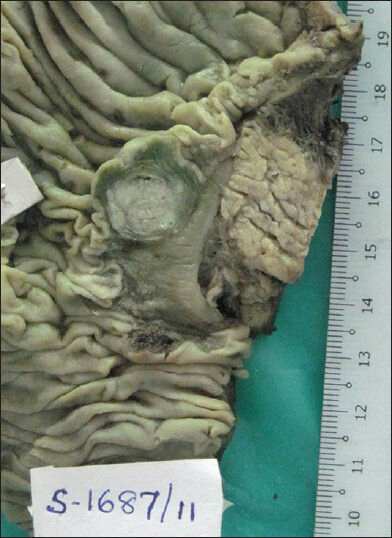
- Gross photograph of periampullary mass measuring 2 × 1 cms in submucosal region
Microscopy showed tumor composed of solid cell nests, cords, ribbons of uniform round to polygonal cells with round regular nucleus and ‘salt and pepper’ chromatin. These cells showed mild pleomorphism and low mitotic rate [Figure 2]. At places cells were arranged in tubuloglandular or acinar architecture distended by mucin. The surrounding pancreas and CBD were uninvolved by the tumor. Out of six lymph nodes, five showed tumor metastasis [Figure 3].
IHC (immunohistochemistry) showed diffuse chromogranin positivity in both classical areas as well as in tubulo-glandular areas and was negative for cytokeratin (CK7 and CK 20). Retrospective analysis of 5 hydroxy indole acetic acid (HIAA) and serotonins were within the normal limits.
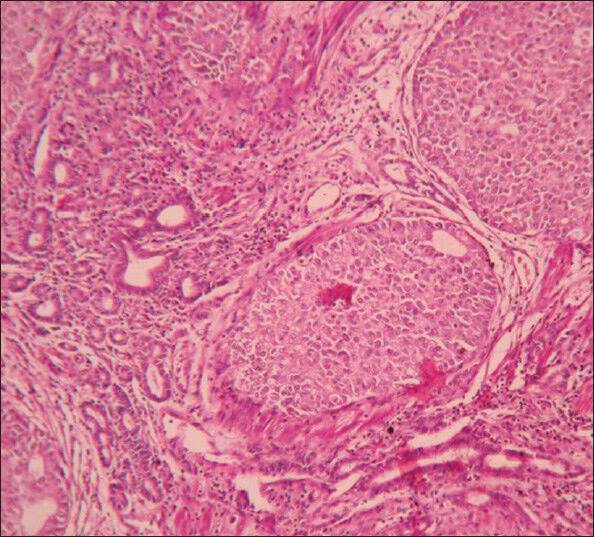
- Microphotograph of tumor displaying both nests and tubular arrangement of cells (×400)
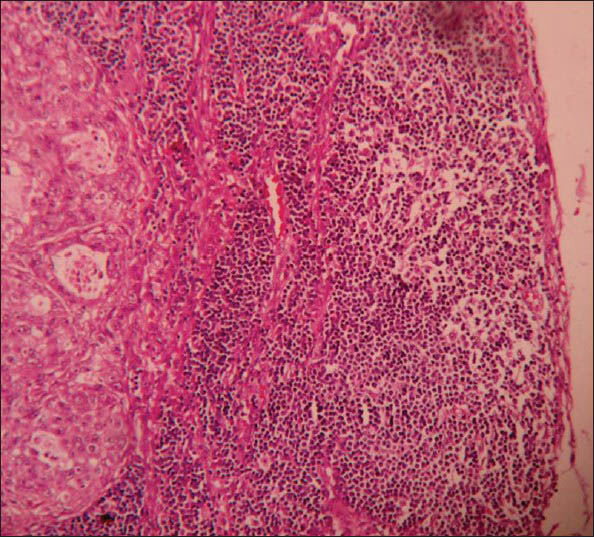
- Microphotograph of peripancreatic lymphnode showing tumor metastasis (×400)
DISCUSSION
In adults the most common cause of periampullary growth are either benign or malignant tumors of duodenum followed by pancreas and rarely tumors of extra hepatic biliary tree.
In the duodenum commonest tumors are carcinoids. They represent 2-3% of all gastrointestinal neuroendocrine tumors in the duodenum. They are most commonly located in the appendix.[4]
Whenever, carcinoids display dual differentiation in the form of typical carcinoids and acinar arrangement it is referred as the adenocarcinoids.[5] The duodenal adenocarcinoids form interesting important group of lesions for several reasons. Their presence especially in periampullary region may cause great confusion as they are mistaken for adenocarcinoma from duodenum, pancreas or extra hepatic biliary tree. They rarely have detectable levels of serotonin unlike the conventional carcinoids.[2]
Unlike the conventional carcinoids perineural invasion and lymphatic spread are unreliable prognostic features. Even the size of the neuroendocrine component does not correlate with biological behavior of the tumor. There are some conflicting reports about nuclear pleomorphism and higher mitotic rate are being considered as prognostic factors in adenocarcinoids.[3]
The natural history of extra appendicial adenocarcinoid is not well known. It is important to recognize these rare occurrences because of different therapeutic approach. The adenocarcinoids are mainly treated with complete surgery without adjuvant chemotherapy whereas the adenocarcinoma requires chemotherapy.[6]
ACKNOWLEDGEMENT
Authors are thankful to Dr. Mahadeva KC Professor and HOD and Dr. Vijaya Mysorekar Professor, Department of pathology for their support.
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- Adenocarcinoids tumor of extra hepatic biliary tree. Int J Surg Pathol. 2008;16:455-57.
- [Google Scholar]
- Duodenal carcinoids: Imaging features with clinical - pathological comparison. Radiology. 2005;237:967-72.
- [Google Scholar]
- Composite (adenocarcinoid) tumor of gastrointestinal tract. Dig Dis Sci. 1990;35:519-25.
- [Google Scholar]
- Carcinoids of ampulla of vater. Clinical characteristics and morphological features. Cancer. 1994;73:1580-8.
- [Google Scholar]
- Collision tumor of the ampulla of Vater: Carcinoid and adenocarcinoma. Rev Esp Enferm Dig. 2007;99:235-8.
- [Google Scholar]
- Tumor of small and large intestine. In: Fletcher CDM, ed. Diagnostic histopathology of tumors (3rd ed). China: Churchill Livingston Elselvier; 2007. p. :379-416.
- [Google Scholar]





