Translate this page into:
Platelet-to-international normalized ratio as a predictor for mortality in moderate to severely ill patients with SARS-CoV-2 infection
*Corresponding author: Deepti Joshi, Department of Pathology and Lab Medicine, All India Institute of Medical Sciences, Bhopal, Madhya Pradesh, India. deepti.patho@aiimsbhopal.edu.in
-
Received: ,
Accepted: ,
How to cite this article: Manohar L, Goel G, Joshi A, Saigal S, Sharma T, Ingle V, et al. Platelet-to-international normalized ratio as a predictor for mortality in moderate to severely ill patients with SARS-CoV-2 infection. J Lab Physicians. 2025;17:53-9. doi: 10.25259/JLP_125_2024
Abstract
Objectives
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has a high mortality and the non-survivors were reported to have a severe form of coagulopathy during the coronavirus disease pandemic. This study was performed to understand hemostatic abnormalities and their relationship to outcomes in patients with moderate to severe SARS-CoV-2 infection. We also evaluated the role of the platelet-to-international normalized ratio (INR) ratio as a predictor of mortality and compared it with other commonly used coagulopathy scores.
Materials and Methods
This study was conducted on all adult patients of SARS-CoV-2 infection who were hospitalized at a tertiary care hospital in Central India between March 1st, 2020, and June 30th, 2021. A cross-sectional study design was used and hemostatic abnormalities among survivors and non-survivors were compared and the role of platelet to INR ratio as a predictor of mortality was evaluated.
Statistical analysis
A descriptive statistical analysis was conducted using the R-Statistical language (version 4.0.3). Appropriate statistical tests were used for comparison and P < 0.05 was used to classify the difference between the two groups as significant. A receiver-operating curve analysis was performed to identify meaningful predictors of mortality.
Results
Of 941 individuals with moderate to severe SARS-CoV-2 infection, included in the study, 291 (30.9%) died. Non-survivors were older and had significantly higher prothrombin time, INR, D-dimer, and activated partial thromboplastin time levels. Platelet-INR ratio was found to have higher predictability for mortality, as compared to disseminated intravascular coagulation and score or sepsis induced coagulopathy scores.
Conclusions
The platelet-INR ratio is an indicator of the severity of abnormal hemostasis abnormality and emerged as a promising biomarker for predicting prognosis in patients with coronavirus disease 2019 pneumonia in the present study.
Keywords
Coagulopathy
Coronavirus disease 2019
Mortality
Platelet-international normalized INR ratio
INTRODUCTION
The modern world recently witnessed one of the worst pandemics of history in the form of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, which resulted in the deaths of millions of individuals all across the globe in 2 years.[1] In its more severe form, it was characterized by massive endothelial cell injury, coagulation activation, excessive immune/inflammatory reaction, cytokine storm, thrombosis, and multiple organ failure. Initial studies revealed a range of hemostatic abnormalities in these patients.[2] This coronavirus disease 2019 (COVID-19) associated coagulopathy (CAC) was found to be different from disseminated intravascular coagulation (DIC) and was characterized by mild thrombocytopenia, mildly increased prothrombin time (PT), and activated partial thromboplastin time (aPTT) but marked increase in fibrin/fibrinogen degradation products (FDP) and D-dimer levels. In comparison, patients with DIC show markedly increased PT and aPTT values along with decreased platelets. CAC occurs due to cytokine storm leading to activation of coagulation cascade whereas in DIC, the coagulation pathway is activated mainly by activation of tissue factors due to varied causes. These studies also reported that abnormal PT, FDP, and D-dimer levels correlated with hospital mortality, and the aberration in the coagulation pathway was significantly associated with multiple indicators of inflammation.[2-4] Hence, a panel of inflammatory and coagulation biomarkers such as C-reactive protein and D-dimer levels were used by the clinicians for risk stratification and optimal utilization of intensive care services. As the pandemic evolved, the role of other biomarkers such as leucocyte count, neutrophillymphocyte ratio (NLR), absolute lymphocyte count, platelet count, and interleukin-6 levels in the assessment of severity and determination of prognosis was also recognized.[5] Some studies have reported differences in hemostatic abnormalities in different waves of the SARS-CoV-2 pandemic.[6]
We performed this study to understand hemostatic abnormalities (platelet count, PT [Prothrombin time and international-normalized ratio (INR)], aPTT, DIC score, or sepsis-induced coagulopathy [SIC] score) and their relationship to outcome in patients with moderate to severe SARS-CoV-2 infection. Since previous literature has identified a fall in platelets and a rise in coagulation parameters, we evaluated a composite measure of platelet-to-INR ratio as a predictor of mortality and compared it with other composite measures such as DIC and SIC scores. We also compared the overall predictive values of coagulation and thrombosis-related variables and performed subgroup analysis for the first and second waves of the pandemic in India.
MATERIALS AND METHODS
We performed a cross-sectional study of all moderate to critically ill adult patients with COVID-19 pneumonia who were hospitalized at All India Institute of Medical Sciences, Bhopal, between March 1st, 2020, and June 30th, 2021. The study design was approved by the Institutional Human Ethics Committee for research on human subjects. All adult patients admitted with SARS-CoV-2 infection, and who had a moderate to severe illness as per clinical criteria were sought to be included. The cases of patients who had mild illnesses and for whom a coagulation profile was not ordered by the clinician were not included in the study. Clinical and demographic details of all the patients fulfilling inclusion criteria were abstracted from the case record forms. Details of oxygenation, ventilatory support required, sequential organ failure assessment (SOFA) SIC score, presence of comorbidities, and outcome (discharge/survival) for critically ill patients were abstracted from the intensive-care unit charts. To estimate the platelet-to-INR ratio, we considered the number of platelets (×103 per cu mm) and INR as an absolute value. Thus, if a patient had a platelet count of 150 × 103 per cu mm, and INR was 1.10 the platelet to INR ratio will be 150/1.1 = 136.36. The role of platelet-to-INR ratio as a predictor of mortality was compared with other commonly used coagulopathy scores in the present study.
Laboratory procedures
Samples for routine coagulation studies were received in a 3.2% sodium citrate vial, with blood to the anticoagulant ratio of 9:1, centrifuged at 3000 rpm for 20 min to yield platelet-poor plasma from which subsequent tests were performed. Complete blood count (CBC) samples were received in an Ethylenediaminetetraacetic acid (EDTA) vial. PT and aPTT were performed in the semi-automated Stago coagulometer (Start Max, France). Fibrinogen and D-dimer were performed on a Stago automated coagulometer (STA Satellite MAX, France). All the tests were performed using propriety reagents (Diagnostica Stago). The samples were processed within 2 h of reception. Appropriate controls were used with all the tests. The samples for CBC were run on Mindray BC-6800 hematology analyzer (Shenzhen, China) using propriety reagents.
Statistical analysis
We compared the clinical characteristics and hemostatic profiles among survivors and non-survivors. Statistical analyses were conducted using the R-Statistical language (version 4.0.3; RCoreTeam, Vienna, Austria) on MacOS Catalina 10.15.6, using the package sggpubr (version 0.4.0), gt summary (version 1.4.1), ggplot2 (version 3.3.2), and tidyverse (version 1.3.0). We performed descriptive statistical analysis for all categorical and quantitative variables. The results were given as the median (inter-quartile range), or number (percentage), wherever appropriate. The difference between continuous and categorical variables was compared using the Wilcoxon rank-sum test and Fisher exact tests/Pearson Chi-square test, respectively, and P < 0.05 was used to classify the difference between the two groups as significant.
RESULTS
A total of 941 samples of individuals with moderate to severe SARS-CoV-2 infection were received in the coagulation laboratory for testing. Of these 695 (73.8%) samples were received during the second wave, when the proportion of more seriously ill patients was also higher. A total of 291 (30.9%) died during hospital stay [Figure 1]. We compared the characteristics of individuals who died with those who had survived. Those who died were older and had significantly higher PT, INR, D-dimer, and aPTT levels. Those who died also had significantly low platelets and higher SOFA scores and SIC scores as compared to survivors [Table 1].
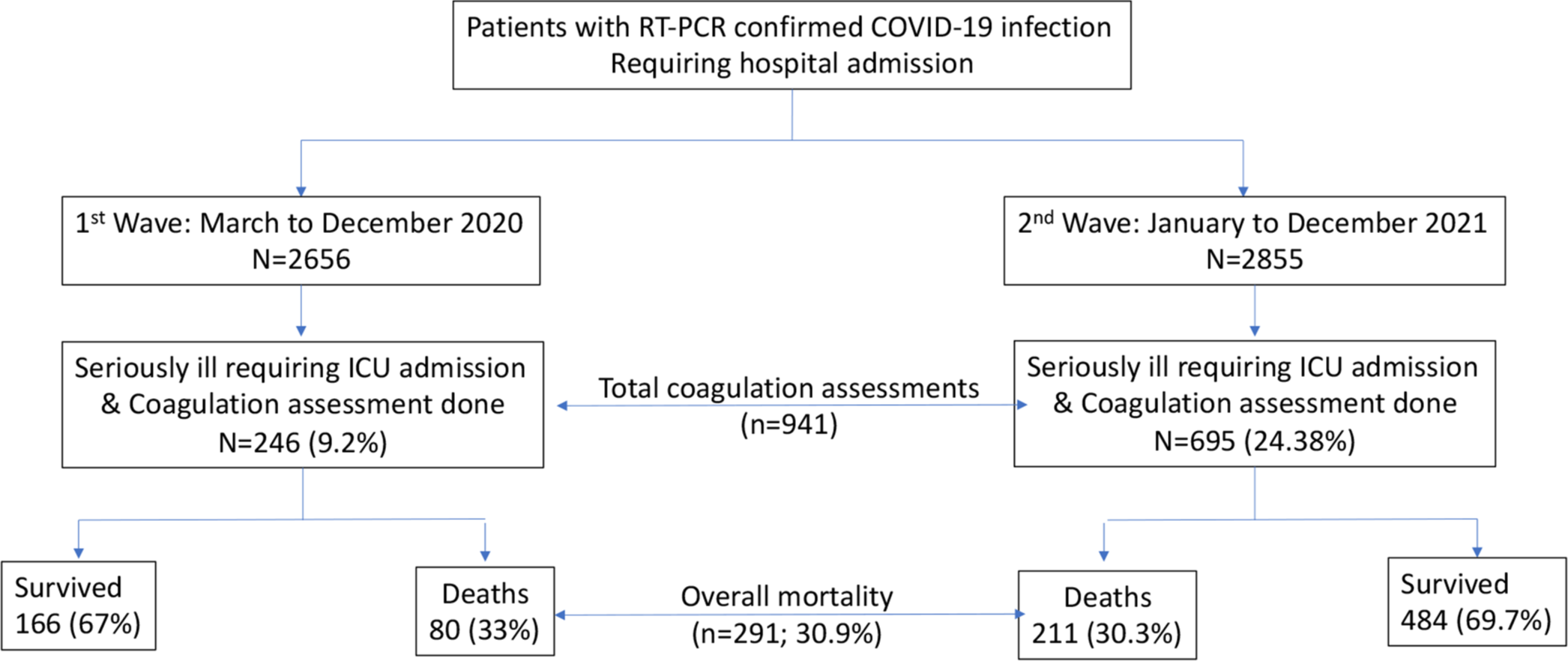
- Study flow, RT- PCR: Reverse transcription-polymerase chain reaction, ICU: Intensive care unit.
| Characteristic | Overall, n=9411 | Survivors n=6501 | Non-survivors n=2911 | P-value2 |
|---|---|---|---|---|
| Mean age (years) | 50 (16) | 47 (16) | 56 (14) | <0.001 |
| Female gender | 280 (30%) | 202 (31%) | 78 (27%) | 0.2 |
| Coagulation factors (mean [SD]) | ||||
| PT (seconds) | 16.9 (6.4) | 15.5 (2.7) | 19.9 (10.1) | <0.001 |
| INR | 1.44 (1.25) | 1.23 (0.35) | 1.91 (2.12) | <0.001 |
| aPTT (seconds) | 31 (13) | 29 (8) | 37 (18) | <0.001 |
| aPTT/aPTT control | 0.98 (0.40) | 0.90 (0.25) | 1.15 (0.59) | <0.001 |
| Fibrinogen levels (mg/dL) | 514 (230) | 480 (206) | 559 (254) | 0.12 |
| D-dimer (Nmol/liter) | 5 (9) | 3 (7) | 8 (11) | <0.001 |
| Platelet count (Thousand/cumm) | 191 (83) | 226 (73) | 112 (36) | <0.001 |
| SOFA score | 1.88 (2.86) | 0.28 (0.98) | 5.46 (2.37) | <0.001 |
| SIC score (%) | ||||
| 3 | 581 (62) | 565 (87) | 16 (5.5) | <0.001 |
| 4 | 213 (23) | 74 (11) | 139 (48) | |
| 5 | 110 (12) | 11 (1.7) | 99 (34) | |
| 6 | 37 (3.9) | 0 (0) | 37 (13) | |
| Platelet-INR ratio | 159 (106,209) | 189 (154,229) | 85 (64,109) | <0.001 |
| Period (%) | ||||
| First wave | 246 (26) | 166 (26) | 80 (27) | 0.5 |
| Second wave | 695 (74) | 484 (74) | 211 (73) | |
We performed receiver-operating curve analysis to identify hemostatic variables that had a better predictive ability for mortality. The area under the curve (AUC) for the PT ratio was 0.66 in the first wave and 0.78 in the second wave. SIC score (which is an additive score for PT-INR, platelet counts, and SOFA) had a higher AUC of 0.84 in the first wave, and 0.96 in the second wave. Since the hallmark of SCI is a fall in platelet counts and a rise in PT-INR, we evaluated the platelet-INR ratio. AUC for this parameter was better than others, 0.94 in the first wave and 0.95 in the second wave [Figure 2]. This ratio was found to be lower amongst survivors as compared to non-survivors [Table 1 and Figure 3].
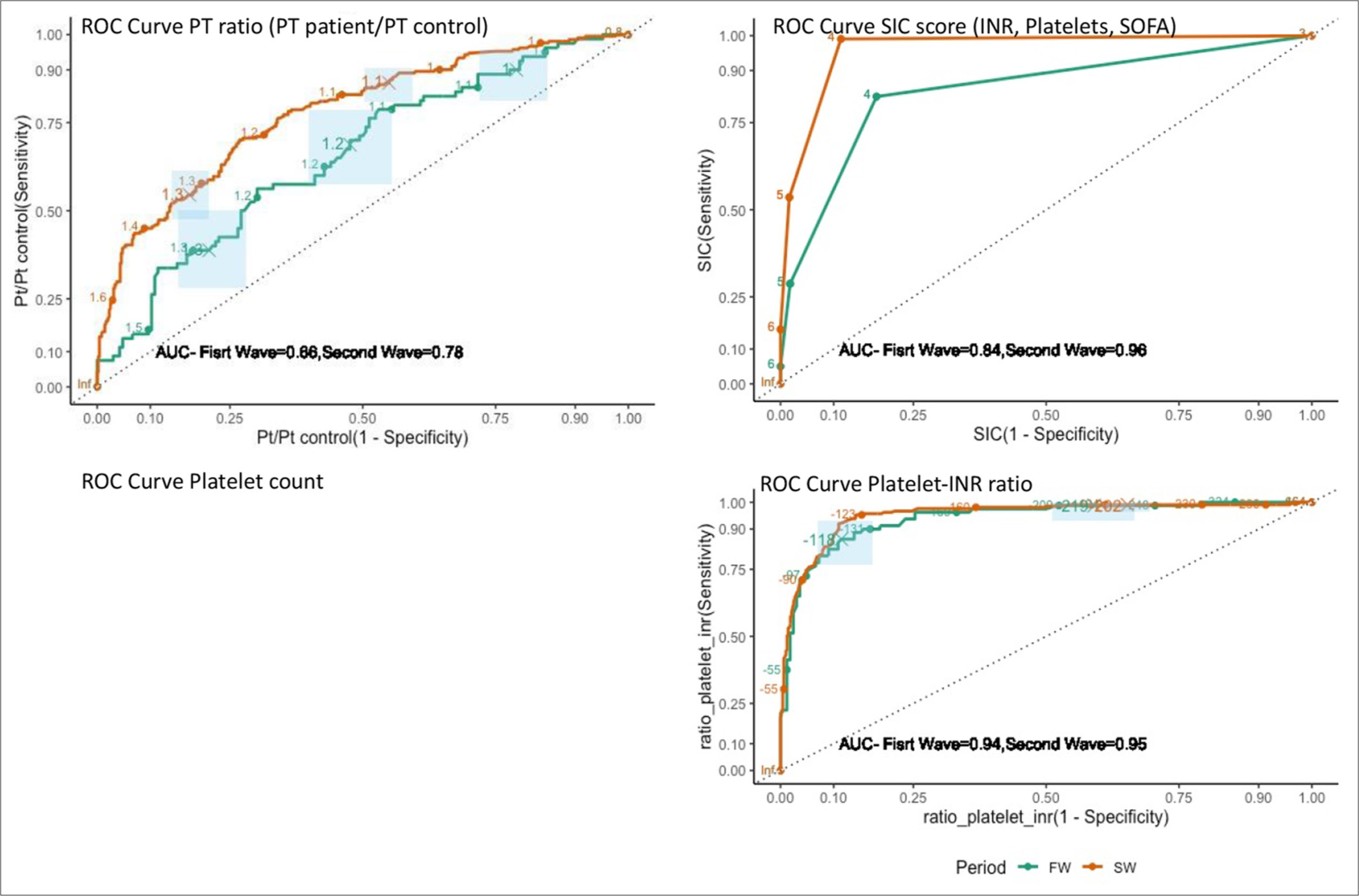
- Receiver operation curve characteristics for coagulation-related indicators for prediction of mortality in 1st and 2nd waves of coronavirus disease 2019 pandemic. FW: First wave, SW: Second wave, PT: Prothrombin time, SIC: Score sepsis-induced coagulopathy score. The ROC curve with the highest AUC is the most predictive of mortality risk. ROC: receiver-operating curve, AUC: Area under the curve, INR: International normalized ratio, SOFA: Sequential organ failure assessment

- Distribution of platelet to the international normalized ratio in survivors and non-survivors as shown in a raincloud plot, INR: International normalized ratio.
DISCUSSION
In our study, we found that the platelet-to-INR ratio had the best predictive ability for mortality, in patients with SARS-CoV-2 infection. Besides this novel ratio, increased age, high SOFA score, high SIC score, elevated PT, INR, aPTT, D-dimer levels, and thrombocytopenia were important predictors of mortality among moderate to severe SARS-CoV-2 infected participants of our study.
Platelet-to-INR ratio combines information from two consequences of hyperinflammation. A fall in platelets is indicative of consumption, usually due to DIC. The same process also causes coagulopathy and a rise in INR. This finding is compatible with the pathophysiology of severe SARS-CoV-2 infection. Now, it is recognized that the disease starts as pulmonary intravascular coagulopathy which may progress to SIC or DIC.[7] It is however debatable if the enhanced coagulation response is a response to a hyperinflammatory state or if the virus directly interfered with the coagulation pathways resulting in systemic thrombosis.
Previous studies have used a reverse INR-platelet ratio to predict liver fibrosis in patients with chronic hepatitis B infection, but to the best of our knowledge, the utility of this ratio has not been yet evaluated for assessing the prognosis of patients with ARDS or SARS-CoV-2 infection.[8] SIC score and DIC score were found useful in predicting mortality due to CAC in few studies but the need for frequent monitoring and interpretation in appropriate clinical context was also noted.[9,10] In the present study, the SIC score was found to be more predictive of mortality during the second wave which had a more severe form of coagulopathy. On the other hand, the platelet-INR ratio was found to be an extremely significant predictor of mortality for both waves, which may be attributed to the fact that in our study population thrombocytopenia, PT ratio and INR were also important independent predictors of mortality.
Most single-center observational studies that explored an association between platelet count and mortality in patients with COVID-19 pneumonia did not report a statistically significant difference between platelet counts of survivors and non-survivors; however, the result of a meta-analysis of selected studies revealed that the difference between the mean pooled platelet counts of survivors and non-survivors was statistically significant.[11,12] The cause of thrombocytopenia is believed to be multifactorial, it may be attributed to cytokine storm, direct inhibition of marrow thrombopoiesis, increased immune-mediated clearance of platelets by newly formed antibodies, or by alteration of pulmonary capillary bed resulting into decreased fragmentation of megakaryocytes eventually leading to decreased number of platelets in circulation. The possibility of consumption coagulopathy and heparin-induced thrombocytopenia also needs to be explored amongst critically ill patients with COVID-19 pneumonia.[13]
In this study, we found a statistically significant difference between PT values, PT ratio, INR, and aPTT among survivors and non-survivors. Most earlier studies had reported only a mild increase in PT among patients with severe disease and had also suggested that such mild prolongations might be missed if ratios are taken into account and had advised the use of absolute PT values.[13,14] Since our sample size was large, our results are similar to those obtained by pooled analysis of various observational studies in a meta-analysis.[12] It is also possible that the patients who were hospitalized in our hospital, especially during the second wave had a more severe form of coagulopathy. The initial studies had also not reported a significantly higher aPTT in patients with COVID-19 infection[15] or found this to be significantly associated with disease severity[16] and it was proposed that though consumption of various coagulation factors occurs in COVID-19 coagulopathy, aPTT is not affected due to increased levels of Factor VIII, which along with factor IX is the major factor which determines the results of aPTT. Factor VIII is also an acute phase reactant and its levels rise in inflammatory conditions.[15] Mild prolongation of aPTT can also be attributed to the development of lupus anticoagulant (LAC). We did not perform factor assays in our study and did not perform mixing studies or screening for LAC, but the significantly increased levels of PT and aPTT amongst non-survivors along with thrombocytopenia is probably indicative of consumption coagulopathy in at least a subset of these patients.
Amongst the various coagulation parameters, the role of D-dimer as a marker of severity of the disease has been most extensively studied and validated. The results of our study are in concordance with all other studies where increased levels of D-dimer were found to be associated with a more severe form of the disease.[13,16] D-dimer is a type of FDP. Its levels are raised in all the stages of CAC. Since there is a lot of thrombin generation both in the lungs as well as in systemic circulation in CAC, the degradation products are increased due to subsequent fibrin breakdown.[3] Excessive fibrin generation in the lung during the initial phases of the disease serves to check the spread of infection, but as the coagulopathy evolves into its systemic phase, the fibrinolytic mechanisms of the body get overwhelmed by this massive fibrin generation and thrombotic complications follow.[3]
In the present study, increased age was a significant predictor of mortality which can be attributed to the fact that people of advanced age are also more likely to have a greater number of comorbidities such as diabetes mellitus and hypertension which are also predictors of mortality in COVID-19 pneumonia.[17]
There has been use of other hematological and coagulation parameters in various studies to determine the severity of COVID-19. In previous studies, the role of NLR and platelet-large cell ratio (P-LCR) in determining mortality in COVID-19 patients has also been reported.[17,18] Higher neutrophilic infiltration in tissues leads to increased generation of cytokines, thereby increasing the severity of the disease. Neutrophilia along with a reduction in absolute lymphocyte number predicted poor survival even among healthy individuals without any associated co-morbidities.[17] P-LCR has also been reported to correlate with the severity of inflammation in all COVID-19 patients, and an elevated P-LCR was considered to be associated with the risk of severe disease and death.[18] We had earlier reported a decline in levels of antithrombin 3 (AT-3) on serial measurements among non-survivors of COVID-19 pneumonia. AT-3 is a potent endogenous anticoagulant and is required for the action of heparin. Decreased AT-3 levels may be attributed to an inflammatory state or consumption coagulopathy among non-survivors. It has also been proposed as a potential biomarker to indicate the severity of the disease.[19]
Our study has several strengths. This is one of the largest studies from India where the role of coagulopathy in determining the outcome of patients with COVID-19 pneumonia has been evaluated. We identified the platelet-INR ratio as a novel prognostic biomarker for predicting the course of CAC. Besides this, the results of our study have provided evidence regarding the role of simple hemostatic parameters such as platelet count, PT, and aPTT in determining the course of the disease besides D-dimer levels.
This study has certain limitations. Since it was a hospital based study, the hemostatic profile of only moderate to critically ill patients was analyzed while the majority of population suffered from a milder form of disease. Results of LAC assays or Factor VIII levels were not available which could have influenced the results of routine coagulation assays. Moreover, viscoelastic assays were not performed which could have provided a global assessment of the coagulation cascade.
CONCLUSIONS
The course of COVID-19-associated pneumonia gets complicated with the progression of CAC. The platelet-INR ratio is an indicator of the severity of coagulopathy and emerged as a promising biomarker for predicting prognosis in patients with COVID-19 pneumonia in the present study. We also found increased age, high SOFA score, high SIC score, elevated PT, INR, aPTT, d-dimer levels, and thrombocytopenia as important predictors of mortality in the present study. Since the platelet-INR ratio is a relatively inexpensive biomarker, the utility of this biomarker for triaging patients in the intensive care unit needs to be further validated by future prospective studies.
Author contribution
SM: Collected data; GG: Conceptulisation of study design; AJ: Stastical analysis; SS: Data collection and review of manuscript; TS: Review of lab procedures, VI data collection; AG: Data collection and manuscript review; RJ: Study design and manuscript; DJ: Conceptulisation of study and overall supervision.
Ethical approval
The research/study was approved by the Institutional Review Board at All India Institute of Medical Sciences, Bhopal, number 2020/pg/July/33, dated 24th February 2021.
Declaration of patient consent
Patient consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- COVID-19 and your health. 2020. Available from: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html [Last accessed on 2023 Dec 28]
- [Google Scholar]
- Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438-40.
- [CrossRef] [PubMed] [Google Scholar]
- Thrombosis and coagulopathy in COVID-19: An illustrated review. Res Pract Thromb Haemost. 2020;4:744-51.
- [CrossRef] [PubMed] [Google Scholar]
- Coagulation abnormalities and thromboprophylaxis in COVID-19. Indian J Med Res. 2021;153:606-18.
- [CrossRef] [PubMed] [Google Scholar]
- Biomarkers in COVID-19: An upto-date review. Front Pediatr. 2020;8:607647.
- [CrossRef] [PubMed] [Google Scholar]
- Second wave of the COVID-19 pandemic: D-dimer levels are not so high anymore. J Thromb Thrombolysis. 2021;52:779-81.
- [CrossRef] [PubMed] [Google Scholar]
- Understanding COVID-19-associated coagulopathy: From PIC to SIC or DIC. J Intensive Med. 2021;1:35-41.
- [CrossRef] [PubMed] [Google Scholar]
- INRto-platelet ratio (INPR) as a novel noninvasive index for predicting liver fibrosis in chronic hepatitis B. Int J Med Sci. 2021;18:1159-66.
- [CrossRef] [PubMed] [Google Scholar]
- Disseminated intravascular coagulation score and sepsis-induced coagulopathy score in prediction of COVID-19 severity: A retrospective analysis. Indian J Crit Care Med Peer-Rev. 2021;25:1357-63.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic and prognostic value of Sepsis-Induced coagulopathy and International Society on Thrombosis and Hemostasis scoring systems in COVID-19-associated disseminated intravascular coagulopathy. J Res Med Sci. 2021;26:102.
- [CrossRef] [PubMed] [Google Scholar]
- Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin Chim Acta. 2020;506:145-8.
- [CrossRef] [PubMed] [Google Scholar]
- Severe COVID-19 and coagulopathy: A systematic review and meta-analysis. Ann Acad Med Singap. 2021;50:325-35.
- [CrossRef] [PubMed] [Google Scholar]
- Hemostatic abnormalities in COVID-19: An update. Indian J Hematol Blood Transfus. 2020;36:616-26.
- [CrossRef] [PubMed] [Google Scholar]
- Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-7.
- [CrossRef] [PubMed] [Google Scholar]
- Heparin dosage, level, and resistance in SARS-CoV2 infected patients in intensive care unit. Int J Lab Hematol. 2021;43:1284-90.
- [CrossRef] [PubMed] [Google Scholar]
- A Review of pathophysiology, clinical features, and management options of COVID-19 associated coagulopathy. Shock. 2021;55:700-16.
- [CrossRef] [PubMed] [Google Scholar]
- Predictors of mortality among hospitalized COVID-19 patients and risk score formulation for prioritizing tertiary care-An experience from South India. PloS One. 2022;17:e0263471.
- [CrossRef] [PubMed] [Google Scholar]
- The role of platelet large cell ratio in determining mortality in COVID-19 patients. Medicine (Baltimore). 2024;103:e38033.
- [CrossRef] [PubMed] [Google Scholar]
- Adequate antithrombin III level predicts survival in severe COVID-19 pneumonia. Cureus. 2021;13:e18538.
- [CrossRef] [Google Scholar]






