Translate this page into:
Predictability of Hematological Parameters in the Diagnosis of Cesarean Scar Pregnancy
Address for correspondence: şukran Dogru, MD, Division of Perinatology, Meram Faculty of Medicine, Necmettin Erbakan University, Konya, Turkey (e-mail: Sukrandogru-2465@hotmail.com).
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction
Cesarean scar pregnancy (CSP) is an increasing clinical condition that causes serious maternal morbidity and mortality. This study aimed to evaluate if inflammation markers measured by hemogram can aid in the diagnosis of CSP.
Materials and Methods
A total of 86 patients were included in the study. The cases were divided as CSP (n: 42) and normal pregnancy (NP) (n: 44). At the time of admission, peripheral blood neutrophils, lymphocytes, monocytes, thrombocytes, systemic inflammatory index (SII) (neutrophil × platelet/lymphocyte), neutrophil–lymphocyte ratio, monocyte–lymphocyte ratio, and platelet–lymphocyte ratio were all measured. CSP and NP diagnoses were made by transabdominal or vaginal ultrasonography.
Results
In the CSP group, mean age (p < 0.001), gravida (p < 0.001), parity (p < 0.001), number of surviving children (p < 0.001), number of abortions (p < 0.001), cesarean number (p < .001), dilatation and curettage count (p = 0.013), monocyte (M) value (p = 0.039) and monocyte/lymphocyte value (MLR) (p = 0.035) were significantly higher than the control group. The optimal M value cut-off value was found to be > 0.40, the sensitivity value was 78.57, and the specificity value was 50.00. AUC = 0.632 (SE = 0.061) for the MLR value. The optimal MLR cut-off value was found to be > 0.232, the sensitivity value was 61.90, and the specificity value was 63.64.
Conclusion
Hemogram parameters, which are simple, inexpensive, and easily accessible, M and MLR are significantly higher in the diagnosis of CSP and can be used as an auxiliary parameter for ultrasonography.
Keywords
cesarean scar pregnancy
hemogram parameters
systemic inflammatory index
Introduction
Cesarean scar pregnancy (CSP) refers to pregnancies that occur in the scar area of a previous cesarean section. Its incidence is increasing all over the world due to the increasing number of cesarean sections in recent years.[1] The true incidence is unknown. According to the literature, its prevalence among all cesarean section patients is estimated to be between 1/1800 and 1/2500. It constitutes 6.1% of all ectopic pregnancies with a history of one or more cesarean sections.[2,3] Increasing awareness among physicians on this issue has increased the incidence rates. Its clinical presentation can be quite variable. Many women are asymptomatic at presentation. Diagnosis is not always simple. Although ultrasonography is the primary diagnostic method, magnetic resonance imaging can assist in some cases.[4] The presence of a pregnancy sac in the lower segment of the first trimester, as well as a history of cesarean delivery, is predictive of the diagnosis. It should be kept in mind that CSP is the precursor of the spectrum of placenta accreta (PAS).
Although the pathogenesis of CSP is unknown, it is known that the nitabuch layer does not develop in the defective decidua, posing a risk for the spectrum of CSP and placenta accreta.[5,6] The pathophysiology of CSP and PAS is known to be the same.[7,8] It is well understood that increased but insufficient trophoblast invasion at the vascularized cesarean section scar causes some inflammatory responses. Recent research has shown that the neutrophil–lymphocyte ratio (NLR), platelet–lymphocyte ratio (PLR), and monocyte–lymphocyte ratio (MLR) can be used as inflammation markers. Neutrophil (N) counts reflect active inflammation, whereas lymphocyte (L) counts regulate this inflammation. While PLR is a thrombosis and inflammation marker, it is also a chronic inflammation marker.[9]
The purpose of this study was to determine whether blood inflammation parameters are effective in predicting and early diagnosing cesarean scar pregnancies, which can be missed and cause serious morbidity and mortality when missed.
Materials and Methods
This study covers the first trimester of CSP and normal pregnancies (NP) followed retrospectively in the Perinatology and Pregnancy Outpatient Clinic of Necmettin Erbakan University (NEU) Meram Medical Faculty Hospital between January 2018 and October 2021. Patients' information was obtained electronically from the NEU Meram Medical Faculty Hospital. Approval for this study was obtained from the NEU ethics committee (decision no: 2022/3577).
A total of 86 patients were included in the study. Demographic data and obstetric histories of all patients were recorded. The patients were divided into two groups CSP and NP patients. Patient numbers were matched one-to-one. The gestational week for both groups was accepted as the first trimester (0–14 weeks). In both groups, those with a history of hyperemesis, imminent abortion diagnosis, twin pregnancy, a history of preeclampsia in a previous pregnancy, those with maternal systemic disease (diabetes, renal diseases, thyroid, heart and blood diseases, chronic hypertension, history of cancer, maternal teratogenic drug including those with autoimmune diseases) and those who smoke and consume alcohol were excluded from the study. Cases were included in the CSP group if its located on the anterior wall of the uterus in the isthmic region, the uterus and cervical canal were empty, and the myometrial thickness was absent or decreased between the bladder and gestational sac, and there was trophoblastic vascular blood flow around the sac. Following diagnosis, dilatation and curettage (D&C) were performed in all these cases. Only the early gestational week with the sac and intrauterine located first-trimester ultrasonography scans with normal fetal heartbeat were included in the NP group. For normal pregnancies, pregnant women with a previous cesarean section history and healthy delivery were randomly included from the electronic record system. All pregnant women had their peripheral venous complete blood count values taken at the time of admission. Hemoglobin (H)(mg/dL), lymphocyte (L)(103/L), neutrophil (N)(103/L), platelet (P)(103/L), and monocytes (M)(103/L) values were calculated, as well as NLR, PLR, MLR, and SII (N × P /L) ratios. Blood samples were collected in sterile ethylenediaminetetraacetate (EDTA) tubes for measurements. All measurements were made using the Mindray BC6200 automated blood count analyzer (Mindray Headquarters, China).
Statistical Analysis
In the descriptive statistics of continuous variables, mean, standard deviation, median, minimum, and maximum values are given in the definition of categorical variables, frequency (n) and percentage (percent) values are given. The normality assumptions of the variables were tested using skewness and kurtosis coefficients, the Kolmogorov–Smirnov test, and the histogram.
The Mann–Whitney U test was performed to compare the non-normally distributed continuous variables between the two groups, and when the normality assumption was met, an independent samples t-test was completed. The variables predicting scar status were determined using logistic regression analysis, and the sensitivity and specificity values were calculated using receiver operating characteristics (ROC) analysis. In all analyses, the IBM SPSS.25 program was used, and p < 0.05 was accepted as the level of significance.
Results
A total of 86 patients, with 44 (51.2%) in the control group and 42 (48.8%) in the scar group, were included in the study. ►Table 1 shows a comparison of the patients included in the study based on obstetric and hematological parameters. As shown in ►Table 1, the mean age of the patients in the scar group (p < 0.001), gravida value (p < 0.001), parity value (p < 0.001), number of surviving children (p < 0.001), number of abortions (p < 0.001), cs number (p < 0.001), dc value (p = 0.013), monocyte value (p = 0.039) and mono/lymph value (p = 0.035) were significantly higher than the control group (►Table 2). The gestational week of the patients in the scar group (p = 0.021) was found to be significantly lower than the control group.
| Parameters | n | Mean ± SD | Median (Min–Max) | p-Value |
|---|---|---|---|---|
| Age* < 0.001 | ||||
| control | 44 | 26.70 ± 5.20 | 27.00 (18.00–38.00) | |
| Scar | 42 | 35.31 ± 5.23 | 34.00 (24.00–49.00) | |
| Gravida** | ||||
| control | 44 | 2.45 ± 1.11 | 2.50 (1.00–5.00) | <.001 |
| Scar | 42 | 3.93 ± 1.50 | 4.00 (2.00–8.00) | |
| Parity** | ||||
| control | 44 | 1.07 ± 0.85 | 1.00 (0.00–3.00) | <.001 |
| Scar | 42 | 2.07 ± 0.75 | 2.00 (1.00–4.00) | |
| Abortion** | ||||
| Control | 44 | .07 ± 0.33 | 0.00 (0.00–2.00) | <.001 |
| Scar | 42 | .83 ± 1.10 | 0.50 (0.00–4.00) | |
| Cesarean** | ||||
| Control | 44 | .52 ± 0.76 | 0.00 (0.00–2.00) | <.001 |
| Scar | 42 | 2.02 ± 0.68 | 2.00 (1.00–3.00) | |
| D&C** | ||||
| Control | 44 | .30 ± 0.51 | 0.00 (0.00–2.00) | .013 |
| Scar | 42 | .76 ± 0.96 | 0.50 (0.00–4.00) | |
| Gestational age** | ||||
| Control | 44 | 7.50 ± 1.17 | 8.00 (5.00–10.00) | .021 |
| Scar | 42 | 6.86 ± 1.69 | 7.00 (4.00–11.00) | |
Abbreviation: D&C, dilatation curettage.
* Independent t-test; **Mann–Whitney U test.
| Parameters | n | Mean ± SD | Median (Min–Max) | p-Value |
|---|---|---|---|---|
| Platelet* | ||||
| Control | 44 | 273.36 ± 56.21 | 269.00 (156.00–459.00) | 0.734 |
| Scar | 42 | 277.76 ± 63.40 | 273.50 (154.00–388.00) | |
| Neutrophil* | ||||
| Control | 44 | 6.36 ± 1.83 | 6.50 (3.50–10.88) | 0.183 |
| Scar | 42 | 6.93 ± 2.15 | 6.75 (2.89–12.91) | |
| Lymphocyte** | ||||
| Control | 44 | 2.12 ± 0.66 | 2.15 (1.25–4.06) | 0.622 |
| Scar | 42 | 2.05 ± 0.69 | 2.08 (0.49–3.74) | |
| Monocyte** | ||||
| Control | 44 | .48 ± 0.17 | 0.41 (0.28–1.06) | 0.039 |
| Scar | 42 | .55 ± 0.24 | 0.51 (0.28–1.80) | |
| Hemoglobin** | ||||
| Control | 44 | 12.83 ± 1.25 | 12.80 (9.20–15.20) | 0.836 |
| Scar | 42 | 12.67 ± 1.50 | 12.80 (8.20–15.90) | |
| Neutrophil/lymphocyte** | ||||
| Control | 44 | 3.20 ± 1.20 | 2.78 (1.59–6.03) | 0.207 |
| Scar | 42 | 4.02 ± 2.71 | 3.25 (1.15–14.04) | |
| Platelet/lymphocyte** | ||||
| Control | 44 | 131.88 ± 37.56 | 128.70 (20.69–206.92) | 0.342 |
| Scar | 42 | 153.41 ± 71.21 | 139.44 (64.29–422.22) | |
| Monocyte/lymphocyte** | ||||
| Control | 44 | .24 ± 0.10 | .21 (0.13 - 0.60) | 0.035 |
| Scar | 42 | .35 ± 0.50 | .26 (0.11–3.33) | |
| Neutrophil x platelet/lymphocyte** | ||||
| Control | 44 | 834.46 ± 341.96 | 752.65 (161.40–1643.77) | 0.099 |
| Scar | 42 | 1093.37 ± 649.09 | 898.78 (185.79–2921.61) | |
* Independent t-test; **Mann–Whitney U test.
To examine the parameters that predict scar condition, a logistic regression analysis was performed. The gestational week was added as a covariate variable to the first step, and monocyte and mono/lymph parameters, which showed significant differences between groups, were added to the second and final steps. As shown in ►Table 3, while the gestational week covariantly predicted scar status significantly (p = 0.03), the monocyte and mono/lymph parameters did not significantly predict the scar status (p > 0.05).
| B | SE | Wald | Exp (B) | p-Value | %95 CI | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Gestational age | −0.335 | 0.162 | 4.261 | 0.716 | 0.039 | 0.521 | 0.983 |
| Monocyte | 1.398 | 1.676 | 0.696 | 4.045 | 0.404 | 0.152 | 107.952 |
| Monocyte/lymphocyte | 2.197 | 2.483 | 0.783 | 8.995 | 0.376 | 0.069 | 1.167.291 |
ROC analysis was used to calculate the diagnostic value by calculating the AUC (area under the ROC curve). AUC = 0.629 (SE = 0.061) for the M value. The optimal M value cut-off value was found to be > 0.40, the sensitivity value was 78.57, and the specificity value was 50.00. AUC = 0.632 (SE = 0.061) for the MLR value. The optimal MLR cut-off value was found to be > 0.232, the sensitivity value was 61.90, and the specificity value was 63.64. ROC curves of M and MLR values are shown in ►Fig. 1 and ►Fig. 2, respectively.
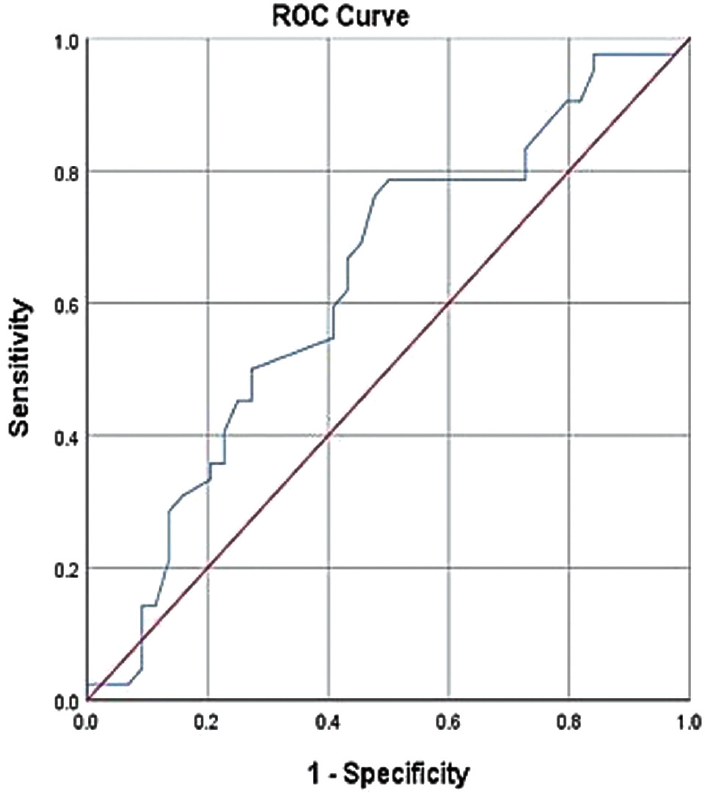
- Receiver operating characteristic curves monocyte for the diagnosis of scar pregnancy.

- Receiver operating characteristic curves monocyte to lymphocyte ratio (MLR) for the diagnosis of scar pregnancy.
Discussion
The purpose of this study was to compare the inflammatory parameters of patients with scar pregnancy to those with normal pregnancy using blood inflammation markers, which have predictive value in many obstetric conditions and gynecological cancers. Although the N, P, and SII rates in scar pregnancies were high, they were not statistically significant. M and MLR were found to be significantly higher. When the gestational age was taken into account, it was discovered that these parameters had no predictive value. This demonstrated that while ultrasonography remains the gold standard in the diagnosis of CSP, blood parameters that are quick, inexpensive, and available everywhere do not aid in the diagnosis.
The use of Doppler with abdominal and vaginal ultrasonography (USG) is still the gold standard for CSP diagnosis. Typical scar pregnancy findings may not always be seen on USG, which may lead to misdiagnosis or delay in diagnosis in CSP, which is the precursor of placental invasion anomalies (PAS). The diagnosis of scar pregnancies is easier between the 5th and 7th gestational weeks than between the 11 and 14th gestational weeks.[10] In one examination of the CSP case series, the mean gestational age at diagnosis was 7.5 ± 2.5 weeks.[11] The diagnosis may be missed in the following weeks of pregnancy because the gestational sac and fetus will grow toward the upper fundus. In this case, close attention should be paid to the placental tissue that remains in the incision line and the vascularization that surrounds it. Differentiating CSP from unavoidable miscarriages and cervical pregnancies is not always simple. Delays in diagnosis can result in uterine rupture and bleeding, which can result in serious maternal morbidity and mortality.[11,12] In a series of 751 CSP cases, 107 (13.6%) underwent hysterectomy because they could not be misdiagnosed or diagnosed, and as a result, these patients lost their fertility.[13] Another study found that 17 (15.4%) of 111 CSP cases were misdiagnosed as an incomplete abortion or cervical pregnancy.[11] Cali et al reported that the lower segment located sac image, which they detected in the first 11 weeks and is the most important finding of CSP in the first trimester, is also a very important finding for PAS in the subsequent weeks.[10] A few CSP cases were followed up on as expectant, and their hysterectomy rates ranged from 50 to 100%, even though PAS was found in almost all of them.[14] This situation necessitates that the physicians involved gain more experience in the diagnosis of CSP.
Recent research on obstetric and gynecological cancers has demonstrated that inflammatory indices obtained in peripheral blood using L, N, M, and P parameters can be an indicator of local and systemic inflammatory response. When these parameters were examined in preeclampsia patients, it was discovered that they could be used to monitor the disease's severity and prognosis. It has been demonstrated that M is elevated in preeclampsia cases and is a good indicator of chronic inflammation, and MLR is a prognostic factor reflecting poor outcomes and body condition.[15] Syncytiotrophoblast microparticles released by the placenta effectively activate neutrophils and stimulate neutrophil formation. It is well known that neutrophils serve as a vital link between syncytiotrophoblasts and vascular endothelial cells and that an increase in N in preeclampsia patients triggers a systemic inflammatory response.[16,17] In third-trimester studies of PAS cases with the same pathophysiology as CSP, NLR was significantly higher than in normal pregnant women, N and PLR ratios were higher, and L ratios were the same.[18–20] In the study conducted by Eskicioglu et al, which compared ectopic and normal pregnancies, N and M values were found to be high, but only M values were found to be statistically significant. PDW (platelet distribution width) is assumed to be low in ectopic pregnancies, M ratios are high, and monocytes may play a role in the pathophysiology of tubal ectopic pregnancies.[21] This result is consistent with the results we found in scar pregnancies, which are considered ectopic pregnancies. Kan et al discovered that NLR and PLR levels were significantly higher in ruptured ectopic pregnancies.[9] In our case series, although L ratios were low and N and P were high, they were not statistically significant. Even though M and MLR values were significantly higher, they were insufficient to establish a cut-off. Perhaps the fact that we did not perform an early D&C due to the risk of complicating pregnancies influenced these results. According to studies, the L ratio is low, and the PLR and NLR are significantly higher in pregnant women with hyperemesis compared with the control group, and these markers can help in the diagnosis.[22]
The limitations of our study include the early detection of scar pregnancies, the early termination of pregnancy, the failure to analyze detailed inflammatory cytokine responses, and the fact that it is retrospective. To examine detailed cytokine response, we believe that laboratory studies and large patient populations are required.
Conclusion
As a result, while systemic inflammatory markers may aid in the diagnosis, they are not predictive. Ultrasonography is an indispensable diagnostic method in the diagnosis of CSP. To avoid fatal complications, public awareness should be raised.
Consent to Publish
The participant has consented to the submission of the case report to the journal.
Informed Consent
Informed consent was obtained from all individuals included in this study.
Ethical Approval
Research involving human subjects complied with all relevant national regulations, institutional policies and is in accordance with the tenets of the Helsinki Declaration (as revised in 2013).
Authors' Contributions
S.D.: study design, patient management, and manuscript writing/editing; F.A.: data analysis, patient management. A.A.A.: data analysis, patient management; A.C.E.: data collection. A.A.: contributed to and approved of the final version of the manuscript.
Acknowledgments
We would like to thank all our colleagues.
Conflict of Interest
None declared.
Funding
None.
References
- Society for Maternal-Fetal Medicine (SMFM)Electronic address: pubs@smfm.org. Society for maternal-fetal medicine (SMFM) consult series# 49: cesarean scar pregnancy. Am J Obstet Gynecol. 2020;222(05):B2-B14.
- [CrossRef] [PubMed] [Google Scholar]
- Placenta accreta: pathogenesis of a 20th century iatrogenic uterine disease. Placenta. 2012;33(04):244-251.
- [CrossRef] [PubMed] [Google Scholar]
- Cesarean scar pregnancy: diagnosis and pathogenesis. Obstet Gynecol Clin North Am. 2019;46(04):797-811.
- [CrossRef] [PubMed] [Google Scholar]
- Limitations of conservative treatment for repeat Cesarean scar pregnancy. Ultrasound Obstet Gynecol. 2005;25(03):310-311.
- [CrossRef] [PubMed] [Google Scholar]
- Caesarean scar pregnancy: a review of management options. Curr Opin Obstet Gynecol. 2011;23(06):415-421.
- [CrossRef] [PubMed] [Google Scholar]
- Pathophysiology of placenta creta: the role of decidua and extravillous trophoblast. Placenta. 2008;29(07):639-645.
- [CrossRef] [PubMed] [Google Scholar]
- Cesarean scar pregnancy is a precursor of morbidly adherent placenta. Ultrasound Obstet Gynecol. 2014;44(03):346-353.
- [CrossRef] [PubMed] [Google Scholar]
- Cesarean scar pregnancy and early placenta accreta share common histology. Ultrasound Obstet Gynecol. 2014;43(04):383-395.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of preoperative neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio on predicting rupture risk in tubal ectopic pregnancies. Gynecol Obstet Invest. 2019;84(04):378-382.
- [CrossRef] [PubMed] [Google Scholar]
- Changes in ultrasonography indicators of abnormally invasive placenta during pregnancy. Int J Gynaecol Obstet. 2018;140(03):319-325.
- [CrossRef] [PubMed] [Google Scholar]
- Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol. 2006;107(06):1373-1381.
- [CrossRef] [PubMed] [Google Scholar]
- Laparoscopic management of an ectopic pregnancy in a previous Caesarean section scar. Hum Reprod. 1999;14(05):1234-1236.
- [CrossRef] [PubMed] [Google Scholar]
- Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. Am J Obstet Gynecol. 2012;207(01):14-29.
- [CrossRef] [PubMed] [Google Scholar]
- Cesarean scar pregnancy: sonographic and magnetic resonance imaging findings, complications, and treatment. J Ultrasound Med. 2012;31(09):1449-1456.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment efficacy of neutrophil-lymphocyte ratio and monocyte-lymphocyte ratio in preeclampsia. J Reprod Immunol. 2019;132:29-34.
- [CrossRef] [PubMed] [Google Scholar]
- Induction of neutrophil extracellular DNA lattices by placental microparticles and IL-8 and their presence in preeclampsia. Hum Immunol. 2005;66(11):1146-1154.
- [CrossRef] [PubMed] [Google Scholar]
- Macrophage-induced apoptosis limits endovascular trophoblast invasion in the uterine wall of preeclamptic women. Lab Invest. 2001;81(08):1143-1152.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic immune-inflammation index predicts prognosis of patients with advanced pancreatic cancer. J Transl Med. 2019;17(01):30.
- [CrossRef] [PubMed] [Google Scholar]
- A cut-off value for systemic immune-inflammation index in the prediction of adverse neonatal outcomes in preterm premature rupture of the membranes. J Obstet Gynaecol Res. 2020;46(08):1333-1341.
- [CrossRef] [PubMed] [Google Scholar]
- Predictive value of complete blood count parameters for placental invasion anomalies. J Matern Fetal Neonatal Med. 2017;30(19):2324-2328.
- [CrossRef] [PubMed] [Google Scholar]
- The efficacy of complete blood count parameters in the diagnosis of tubal ectopic pregnancy. Ginekol Pol. 2014;85(11):823-827.
- [CrossRef] [PubMed] [Google Scholar]
- Subclinical inflammation markers in hyperemesis gravidarum and ketonuria: a case-control study. J Lab Physicians. 2019;11(02):149-153.
- [CrossRef] [PubMed] [Google Scholar]






