Translate this page into:
Presence of Concurrent Derangements of Liver Function Tests in Type 2 Diabetes and Their Relationship with Glycemic Status: A Retrospective Observational Study from Meghalaya
Address for correspondence: Dr. Alice Abraham Ruram, E-mail: ruramalice9@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution NonCommercial ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background and Objectives:
The liver plays a pivotal role in carbohydrate metabolism. Therefore, functional state of the liver in patients with diabetes is of interest. The objectives of the current study were to (i) identify co-existent biochemical derangements of liver function tests (LFTs) in type 2 diabetes and (ii) determine the association between liver function parameters and glycemic status in type 2 diabetics from Shillong, Meghalaya.
Materials and Methods:
Data from 320 type 2 diabetes patients were screened retrospectively for abnormalities in LFTs. Relationship of fasting serum glucose was assessed with the following tests in the LFT panel: Total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and albumin. Correlation coefficient was computed between individual LFT and fasting glucose status. These bivariate analyses were supplemented by multivariate linear regression analyses.
Results:
71.25% subjects had an abnormality in at least one LFT. Elevated ALT (46.8%) and elevated ALP (48.5%) were the most common abnormality in males and females, respectively. ALP correlated positively with fasting glucose in both sexes. AST, ALT, and ALP were found to be independent determinants of glycemic status.
Conclusion:
Derangements in liver function are widely co-existent in type 2 diabetics from Shillong. Deranged liver enzymes are associated with glycemic status. Screening for liver dysfunction in diabetics and subsequent workup may lead to the identification of hepatic co-morbidities and better management.
Keywords
Liver enzymes
liver function
Meghalaya
type 2 diabetes mellitus
INTRODUCTION
Diabetes mellitus (DM) refers to a group of metabolic disorders characterized by hyperglycemia with disturbances in carbohydrate, lipid, and protein metabolism due to defects in insulin secretion, insulin action or both. Type 2 DM is the more prevalent variety of DM caused by resistance to insulin action and inadequate compensatory insulin secretory response.[1] The liver plays a central role in maintaining glucose homeostasis since it extracts glucose from blood to use as fuel, stores dietary glucose as glycogen for later use and also synthesizes glucose from noncarbohydrate sources to maintain blood glucose level during fasting states.[2] Diabetes care is by and large provided by primary care physicians.[3] However, in spite of the manifold roles of the liver in regulating blood glucose levels, evaluation of hepatic involvement features rather infrequently in the workup strategies of primary care providers while dealing with diabetes as opposed to other more frequently featured systemic involvements of the disease such as neuropathy, nephropathy, retinopathy, or cardiovascular involvement.
Liver function tests (LFTs) are one of the most widely requested biochemical test panels by clinicians which provide information about the functional state of a subject's liver. Liver function has been described in the setting of clinical diabetes in the past.[45] More recent studies indicate that altered LFTs, particularly the derangements in the level of liver enzymes such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and gamma-glutamyl transpeptidase (GGT) in the serum are a common occurrence in type 2 DM.[6789] This has attracted considerable attention. In fact, it has been suggested that serum concentrations of liver enzymes are strongly associated with glycemic status and/or insulin resistance.[101112] There is a growing body of evidence that elevated liver enzymes have the potential to serve as strong risk factors for the development of type 2 DM and improve the predictive utility for future type 2 DM when included along with the traditional risk factors.[13141516]
With the dubious honor of being “the diabetes capital of the world,” India is undergoing an alarming trend with the total number of diabetic subjects estimated to rise to 79.4 million by the year 2030.[17] Some studies have reported biochemical alterations in LFTs in relation to type 2 DM in certain parts of India.[18192021] However, most of these studies have been limited by small sample size that precludes robust statistical analyses. There is a dearth of data on the status of liver function in diabetic patients from the northeast Indian state of Meghalaya. This exploratory study was taken up with the chief objective of identifying the biochemical derangements in LFTs in type 2 DM patients from Shillong, Meghalaya. In addition, we analyzed the association of these derangements with the glycemic status.
MATERIALS AND METHODS
Study design
This study was conducted at North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences in Shillong, Meghalaya.
Data from type 2 DM patients diagnosed between December 2014 and March 2015 were retrospectively reviewed. Reports of outpatient fasting serum glucose and LFTs (which included serum levels of total bilirubin, AST, ALT, ALP, and albumin) were compiled. LFTs were advised to these patients as a part of routine screening. Pregnant cases were not included. Individuals who sought medical care primarily for hepatic ailments, and in whom diabetes was detected incidentally were also excluded. A total of 320 reports were selected for the final analysis in this manner.
Liver function tests estimations
The LFTs were estimated in Beckman Coulter AU2700 Plus autoanalyzer using commercially available kits (Beckman Coulter, USA). Quality control was done to ensure the validity of the test results using third party control materials (Randox, UK and Christian Medical College, Vellore). The normal reference ranges used for this study were as follows: Total bilirubin: 0.3–1 mg/dL, ALT: 0–35 U/L, AST: 0–35 U/L, ALP: 30–120 U/L, and albumin: 3.5–5.5 g/dL.[22] The serum levels of total bilirubin, AST, ALT, and ALP were considered deranged when they were more than the respective reference ranges while the levels of albumin were considered deranged when they were less than the reference range.
Ethics
Since the data were collected by retrospective review of the database, hence, there was no scope for obtaining informed consent. The information was de-identified and anonymized, and the study complied with the guidelines of Declaration of Helsinki.
Statistical analysis
Categorical data were expressed as proportions. The continuous data were expressed as a mean ± standard deviation. Tables were prepared in Microsoft Excel 2007 worksheets, and the statistical analyses were performed using GraphPad InStat version 3.00 (GraphPad Software, Inc., California, USA).
The Kolmogorov–Smirnov test was performed to check the data for normal distribution. As the data were not normally distributed, therefore, continuous data between males and females were compared by Mann–Whitney test. Comparison of proportion between males and females was done by using the Chi-square test. Spearman nonparametric correlation coefficient (r) between fasting serum glucose and components of LFT were estimated. Multivariate analyses were performed to assess the individual associations between glycemic status and LFTs. Multiple linear regression models were formed using fasting serum glucose as the dependent variable, while, the LFT parameters and age were used as independent variables. The analyses were done on the overall sample and also separately for males and females.
A two-tailed P < 0.05 was considered statistically significant, whereas a P < 0.01 was considered highly significant.
RESULTS AND OBSERVATIONS
Data were retrieved from 320 subjects of which 188 were males (58.75%), and 132 were females (41.25%). The ages of the subjects ranged from 32 to 69 years.
It was seen that of the total 320 reports, results of LFTs in 92 subjects (28.75%) were within the normal reference ranges. The remaining 228 subjects (71.25%) had an abnormality in at least one liver function parameter. These 228 subjects with deranged LFTs exhibited an elevation in at least one of the tested liver enzymes (i.e. AST, ALT or ALP). Thus, the frequency of altered liver enzymes in the study sample was found to be 71.25%. Of these, 136 were males, and 92 were females. Continuous data on the biochemical parameters are shown in Table 1. Since the values differed significantly between the males and females, subsequent analyses were carried out in a gender-wise manner.

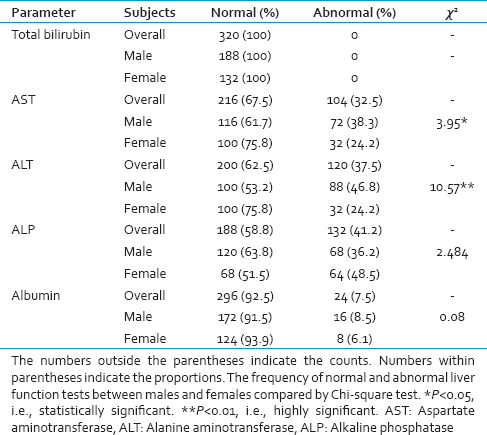
The frequency of normal and deranged results of the individual LFTs are presented in Table 2. All the subjects had normal values for total bilirubin. Overall, the most commonly encountered abnormality of LFT was that of increased ALP (41.2%), while, decreased albumin (7.5%) was the least frequent abnormality. The frequencies of elevated AST, elevated ALT, elevated ALP, and diminished albumin were 32.5%, 24.5%, 41.2%, and 7.5%, respectively. Elevated ALT was the most common abnormality in males (46.8%), whereas, elevated ALP was the most common abnormality in females (48.5%). Decreased albumin was the least common abnormality in both the sexes.
The concentrations of ALP correlated significantly with the fasting serum glucose levels [Table 3]. The correlation was significantly positive in both males (r = 0.36, P < 0.05) and females (r = 0.38, P < 0.05). For the other components of LFTs, none of the correlations were statistically significant (P > 0.05).

The results of multivariate analyses are presented in Table 4. Total serum bilirubin was excluded from the regression models as none of the subjects had abnormal values for serum bilirubin. The multiple linear regression model using AST, ALT, ALP, albumin and age as the independent variables predicted 17.9% of the variation in fasting glucose levels (R2 = 0.179, P < 0.01) in the overall sample. AST, ALT, and ALP were found to be independently associated with fasting glucose values. In the gender-wise analysis, the regression model predicted 15.7% of the variation in fasting glucose (R2 = 0.157, P < 0.01) in males. Likewise, the regression model in females explained 22.6% of the variation in fasting glucose concentrations (R2 = 0.226, P < 0.01). The three liver enzymes (AST, ALT, and ALP) were independently associated with the fasting glucose levels in females. In males, only ALT and ALP were independently associated with fasting glucose values. Age and albumin were not found to be independent predictors in either males or females or in the overall sample.

DISCUSSION
A very high prevalence of abnormal LFTs (71.25%) was observed in patients with type 2 DM in the current study. ALP was the most commonly affected liver function parameter. This was followed by abnormalities in AST, ALT, and albumin in that order. The results in our study were similar to those reported in other populations where ALP was highlighted as the most commonly elevated liver enzyme in diabetics.[923] However, this is not a universal finding, and some other studies have detected abnormalities in the values of aminotransferases (i.e. AST and/or ALT) to be the most commonly deranged LFT in type 2 diabetes.[5724]
We observed that the proportion of abnormal liver functions tests varied in a gender-wise manner. In females, ALP was the most commonly affected parameter. On the other hand, in males, the most commonly deranged parameter was ALT. Furthermore, the proportion of normal and abnormal values for the enzymes AST and ALT varied significantly between males and females. However, no comparison of our findings could be made with those from other studies[724] in this regard due to a lack of data on the gender-wise prevalence of these parameters. Besides, ALP was not estimated as a part of liver function evaluation in a number of previous studies.[681025]
It is increasingly believed that abnormal hepatocellular functions are strongly related to type 2 DM. Prospective studies suggest that abnormality in LFTs is a strong risk factor for the development of diabetes in later life.[131416] Analytical studies have linked elevated liver enzymes with hyperglycemia and/or insulin resistance.[1112] Abnormal LFTs in type 2 DM have also been attributed to factors like nonalcoholic fatty liver disease[2627] and underlying hepatitis C infection.[28] Our study reaffirms the close relationship between type 2 DM and liver dysfunction. The multiple regression models showed that the LFTs predicted the fasting glucose status significantly in both males and females. The liver enzymes were found to be independently associated with the fasting serum glucose levels.
Our study has found a high prevalence (71.25%) of deranged LFTs in type 2 diabetics from Shillong. Earlier studies have indicated that abnormalities in LFTs are not an infrequent occurrence. In Indian diabetics, deranged LFTs have been reported at a frequency of 50–70%.[18192021] This is remarkably higher than the values from Europe and the United States, where derangements in LFTs in diabetics have been reported in the range of 7.8–22.9%.[26] The considerably high frequency of deranged LFTs in type 2 diabetics in the current study and other Indian studies is possible because of co-existence of conditions such as alcoholic liver disease, nonalcoholic fatty liver disease, and other chronic liver diseases. Distinct sampling techniques and use of different reference ranges may be other important reasons for these differences between the Indian and non-Indian studies. Our findings underscore the importance of hepatic function monitoring and subsequent workup in diabetics. The current cohort of diabetic subjects did not seek medical attention for hepatic problems. Liver dysfunction assessment often does not figure in the workup of diabetic patients as opposed to assessment of the involvement of other organ systems such as the heart, nervous system, retina, or the kidneys. This is especially true in the primary care setting, which caters to a large chunk of the diabetic patients.[3] However, a high co-existence of liver function derangements in type 2 diabetics, as seen in our study, has important implications because it may influence prognosis and clinical outcomes, and improve patient care. Detailed workup of such patients may offer opportunities for hepatic case finding. Further, the fact that many anti-diabetic agents have hepatotoxic effects and that liver diseases modulate insulin sensitivity[293031] may necessitate adapting the management protocol keeping in view the hepatic derangements. Moreover, as several liver function parameters were found to be independently associated with fasting glucose levels in our study, it would be interesting to note if improvements in the LFTs would bring about better glycemic control in type 2 diabetics.
Our study had a limitation. Since we conducted the study retrospectively, hence, information on the details of alcohol use was not available. Similarly, information from additional investigations such as serum GGT, ultrasound abdomen and hepatitis virus screen also could not be collated. Although the patients did not seek medical attention for hepatic complaints, yet, these additional data could have provided clues about the etiology of the liver function derangements in our diabetic subjects.
CONCLUSION
The current study highlights the importance of liver function monitoring in patients with type 2 DM. It reveals widely co-existent derangements in LFTs in the type 2 diabetics from Shillong, Meghalaya. To the best of our knowledge, this is the first report of the abnormalities of LFTs in diabetic patients from northeast India. Liver dysfunction in diabetics may denote the presence of other co-morbid conditions. It may facilitate opportunistic case finding. A systematic workup in such patients may offer benefits in terms of improved treatment outcomes and prognosis. Another aspect to these findings is that the liver may be intimately involved in the pathogenesis of type 2 DM and LFTs may serve as valuable indicators for the risk and progression of future diabetes. Future attempts should be directed toward unraveling the causes of hepatic dysfunction in diabetics and examining the impact that hepatic involvement might have on the glycemic status. It is hoped that the current study will encourage in-depth research on liver dysfunction in the context of DM.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors thank doctors Vinay Upadhyay and Binod Gohain for their help.
REFERENCES
- American Diabetic Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2008;31(Suppl 1):S62-7.
- [Google Scholar]
- Chapter: Liver disease. In: Burtis CA, Ashwood ER, Bruns DE, eds. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics (5th ed., 1st Indian Reprint). India: Elsevier (Reed Elsevier India Private Limited); 2012. p. :1637-93.
- [Google Scholar]
- The role of primary care professionals in managing diabetes. Clin Diabetes. 2002;20:65-6.
- [Google Scholar]
- Diabetes mellitus and liver dysfunction; etiologic and therapeutic considerations. Am J Med. 1950;8:290-9.
- [Google Scholar]
- Raised liver enzymes in newly diagnosed Type 2 diabetes are associated with weight and lipids, but not glycaemic control. Indian J Endocrinol Metab. 2012;16:1012-4.
- [Google Scholar]
- Determinants of abnormal liver function tests in diabetes patients in Myanmar. Int J Diabetes Res. 2012;1:36-41.
- [Google Scholar]
- Prevalence of elevated liver enzymes in Type 2 diabetes mellitus and its association with the metabolic syndrome. J Endocrinol Invest. 2008;31:146-52.
- [Google Scholar]
- Alkaline phosphatase: Can it be considered as an indicator of liver fibrosis in non-alcoholic steatohepatitis with type 2 diabetes? Bratisl Lek Listy. 2011;112:626-9.
- [Google Scholar]
- Liver enzymes, type 2 diabetes, and metabolic syndrome in middle-aged, urban Chinese men. Metab Syndr Relat Disord. 2011;9:305-11.
- [Google Scholar]
- One-hour post-load plasma glucose levels are associated with elevated liver enzymes. Nutr Metab Cardiovasc Dis. 2011;21:713-8.
- [Google Scholar]
- Positive correlations of liver enzymes with metabolic syndrome including insulin resistance in newly diagnosed type 2 diabetes mellitus. Endocrine. 2010;38:181-7.
- [Google Scholar]
- Liver enzymes as a predictor for incident diabetes in a Japanese population: The Hisayama study. Obesity (Silver Spring). 2007;15:1841-50.
- [Google Scholar]
- Liver function tests and risk prediction of incident type 2 diabetes: Evaluation in two independent cohorts. PLoS One. 2012;7:e51496.
- [Google Scholar]
- Liver aminotransferases and risk of incident type 2 diabetes: A systematic review and meta-analysis. Am J Epidemiol. 2013;178:159-71.
- [Google Scholar]
- Use of multiple metabolic and genetic markers to improve the prediction of type 2 diabetes: The EPIC-Potsdam Study. Diabetes Care. 2009;32:2116-9.
- [Google Scholar]
- Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047-53.
- [Google Scholar]
- Hepatic dysfunction in diabetes mellitus: Biochemical and ultrasonological study. J Acad Ind Res. 2014;3:164-7.
- [Google Scholar]
- Alarming high levels of transaminases in non-insulin dependent diabetes mellitus. Indian J Basic Appl Med Res. 2014;3:544-8.
- [Google Scholar]
- Liver function tests in type 2 diabetes mellitus patients with and without oral hypoglycemic agents and statin intake. Indian Med Gaz. 2012;10:388-93.
- [Google Scholar]
- Liver dysfunction in diabetes patients admitted in referral hospital. Bali Med J. 2014;3:122-4.
- [Google Scholar]
- Appendices: Laboratory values of clinical importance. In: Kasper DL, Fauci AS, Longo DL, Braunwald E, Hauser SL, Jameson JL, eds. Harrison's Principles of Internal Medicine (16th ed). The United States of America: The McGraw-Hill Companies, Inc.; 2005. p. :A1-7.
- [Google Scholar]
- Elevation of serum alkaline phosphatase activity and related enzymes in diabetes mellitus. Clin Biochem. 1977;10:8-11.
- [Google Scholar]
- Prevalence of abnormal liver function tests in patients with diabetes mellitus. Endocr Abstr. 2007;13:157.
- [Google Scholar]
- Liver function tests in type 2 Sudanese diabetic patients. Int J Nutr Metab. 2011;3:17-21.
- [Google Scholar]
- Study of prevalence of nonalcoholic fatty liver disease (NAFLD) in type 2 diabetes patients in India (SPRINT) J Assoc Physicians India. 2013;61:448-53.
- [Google Scholar]
- Liver disease and diabetes: Association, pathophysiology, and management. Diabetes Res Clin Pract. 2014;104:53-62.
- [Google Scholar]
- Diabetes co-existing with chronic liver disease: Clinical features and response to therapy. Niger J Med. 2007;16:156-60.
- [Google Scholar]





