Translate this page into:
Prevalence of Mupirocin Resistance Among Staphylococci, its Clinical Significance and Relationship to Clinical Use
Address for correspondence: Dr. Manohar Shoorashetty Rudresh, E-mail: rudreshsm@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Mupirocin competitively inhibits bacterial isoleucyl transfer-RNA synthetase and inhibit bacterial protein synthesis. Widespread usage and over the counter availability of the drug has resulted in resistance among Staphylococcus species.
Objectives:
This study aimed to determine the overall prevalence of mupirocin resistance among staphylococci. Correlate clinical significance of mupirocin resistance and its relationship to clinical use.
Methods:
Consecutive, nonrepetitive, clinical isolates of Staphylococcus aureus (n = 98), and coagulase-negative staphylococci (CoNS) (n = 45) from skin and soft-tissue infections between January 2014 and June 2014 were studied. Antibiotic susceptibility testing was done according to Clinical and Laboratory Standards Institute guidelines. Low- and high-level mupirocin resistance was screened by using 5 µg and 200 µg discs respectively and confirmed by agar dilution. Annual consumption of mupirocin was studied and correlated with resistance.
Results:
High-level mupirocin resistance was found in 8.2% S. aureus and 15.6% of CoNS, while low-level mupirocin resistance was found in 17% S. aureus and 8.9% CoNS. High-level mupirocin resistance was more common in methicillin-sensitive S. aureus isolates when compared with methicillin-resistant S. aureus isolates (P < 0.05). Mupirocin resistant S. epidermidis were associated with methicillin resistance and constitutive clindamycin resistance.
Conclusion:
High prevalence of mupirocin resistance was found in the present study. Increased prevalence of mupirocin resistance among community-acquired staphylococci demands the judicious use of the drug in the community.
Keywords
Coagulase-negative staphylococci
methicillin resistant Staphylococcus aureus
methicillin sensitive Staphylococcus aureus
mupirocin resistance
Staphylococus aureus
INTRODUCTION
Mupirocin (pseudomonic acid A) an analogue of amino acid isoleucine is derived from Pseudomonas fluorescens. It competes with isoleucine for binding sites of bacterial isoleucyl transfer RNA (tRNA) synthetase and thereby inhibits protein synthesis.[1] The drug is widely used as a topical antimicrobial agent to treat Staphylococcus aureus infections and carrier status. Widespread usage in clinical practice and over-the-counter availability has led to the development of resistance to this drug.
Mupirocin susceptibility is categorized into three types: Mupirocin susceptible with minimum inhibitory concentration (MIC) of ≤4 µg/ml (MupS), low level resistance (MupRL) with MIC of ≥8–256µg/ml and high level resistance (MupRH) with MIC of ≥ 512 µg/ml. Some authors describe low-level resistance as MIC ≥ 8–64 µg/ml because isolates with MIC of 128 and 256 µg/ml are uncommon.[2] The resistance can be detected in Kirby–Bauer disc diffusion testing by using 5 µg and 200 µg discs. The dilution method is considered the gold standard for the determination of mupirocin-resistance levels.[3]
Coagulase-negative staphylococci (CoNS) are considered to be a part of the normal human flora. However in recent days, CoNS have emerged as a major cause of device associated infections, infections among immunocompromised and cancer patients. Multidrug-resistant phenotypes are more common among CoNS when compared to S. aureus. Many resistances have been proved to originate from CoNS and spread to S. aureus by horizontal gene transfer. Being the reservoir of conjugative plasmids CoNS might have transferred high-level mupirocin resistance to S. aureus.[4]
Mupirocin is mainly used for decolonization of methicillin-resistant S. aureus (MRSA) among patients and health care workers. This selective use has made a higher prevalence of mupirocin resistance among MRSA when compared to methicillin-sensitive S. aureus (MSSA). Higher prevalence of MupRH may also be attributed to selective reports from MRSA outbreaks from hospitals. Studies defining the overall prevalence of mupirocin resistance among staphylococcal isolates are limited.[4]
The present study aimed to determine the overall prevalence of high-and low-level mupirocin resistance among S. aureus and CoNS and correlated with its clinical use.
METHODS
Collection of bacterial isolates
A total of 143 consecutive, nonrepetitive, clinical isolates of S. aureus (n = 98) and CoNS (n = 45) isolated from skin and soft tissue infection from patients of Intensive Care Unit (ICU), pediatric ICU, inpatient, and outpatient departments between January 2014 and June 2014 were included in the study. The isolates were identified as S. aureus and CoNS by standard laboratory techniques. Infectivity of CoNS was established by repeated isolation of same species from two different occasions. CoNS were speciated according to De Paulis et al.[5]
Antibiotic susceptibility testing
The antibiotic susceptibility testing was done by Clinical and Laboratory Standards Institute (CLSI) recommended Kirby–Bauer disc diffusion testing on Mueller-Hinton agar with a panel of 12 antimicrobial agents.[6] Quality control was achieved by using S. aureus ATCC 25923. Inducible and constitutive clindamycin resistance (iMLSB and cMLSB) was determined by placing erythromycin (15 µg) and clindamycin (2 µg) discs placed 15 mm apart. Methicillin resistance was detected by using cefoxitin disc (30 µg) along with routine sensitivity testing.
Disc diffusion method
Pure form of mupirocin was purchased from HiMedia laboratories Pvt Ltd (Mumbai, India). Mupirocin disc of 5 µg and 200 µg strength were prepared in house. The discs once prepared were used within a week. Both discs were included in the routine sensitivity testing and plates were incubated for 24 h at 35 ± 2°C. The zone diameters were carefully examined with transmitted light for light growth within the zone of inhibition. Isolates with no zone of inhibition were interpreted as mupirocin resistant. Isolate resistant to 5 µg disc and any zone for 200 µg disc was considered MupRL. Isolates resistant for both the discs were considered high-level mupirocin resistant[3] [Figure 1].
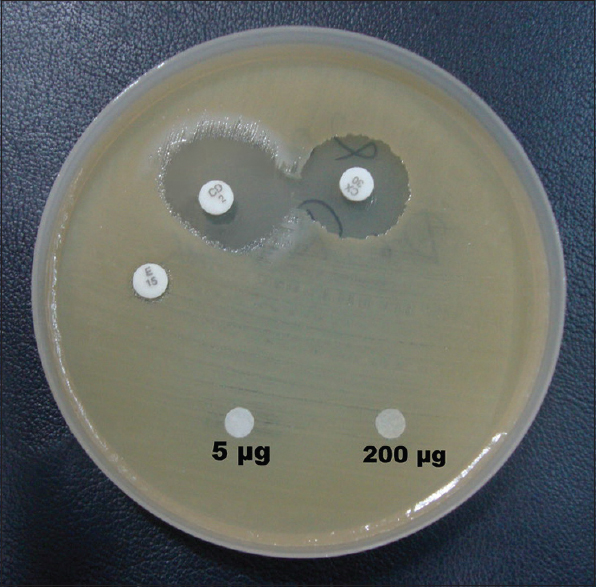
- High level mupirocin resistant methicillin sensitive Staphylococcus aureus showing inducible clindamycin resistance phenotype
Agar dilution method
Minimum inhibitory concentration was detected by CLSI recommended agar dilution method using Mueller-Hinton agar with mupirocin concentration ranging from 0.016 to 1024 µg/ml.[7] Staphylococci with MIC of ≤4 µg/ml were considered MupS, those with 8–256 µg/ml were considered MupRL and isolates with ≥ 512 µg/ml were considered MupRH. Quality control was achieved by S. aureus ATCC 25923. The acceptable range of MIC for control strain was from 0.12 to 0.5 µg/ml.
Statistical analysis
Statistical analysis was performed using Epi infoTM 7.1.4 software program developed by Centers for Disease Control and Prevention (CDC) in Atlanta, Georgia (USA). Simple frequencies were tabulated. Chi-square test was done to determine the statistical significance. P < 0.05 was considered as statistically significant.
RESULTS
A total of 98 (68.5%) S. aureus and 45 (31.5%) CoNS were grown. Among the forty-five CoNS spp., 39 were S epidermidis, 4 were S haemolyticus and 2 were S lugdanensis. A total of 82 staphylococci were isolated from samples of outpatients, 56 from inpatients and 5 from ICU.
Methicillin resistance was found in 22.4% of S. aureus and 20% of CoNS. Methicillin resistance among staphylococci isolated from outpatients and in patients was 19.5% and 24.6%, respectively. iMLSB and cMLSB were found in 19% and 1% of S. aureus. CoNS had higher proportion of cMLSB (22%) when compared to iMLSB (12%).
Overall prevalence of MupRL and MupRH among staphylococci was found to be 14.7% (n = 21) and 10.5% (n = 15), respectively [Table 1]. Fifteen percent of CoNS had MupRH which is very high when compared to the resistance in S. aureus (8.1%). Higher proportion of S. aureus (17.3%) had MupRL resistance when compared to CoNS spp. (8.9%). Among the coagulase negative species, MupRH was found only in S. epidermidis. Of the 7 MupRH S. epidermidis, 5 were methicillin resistant, and four were cMLSB [Table 2].


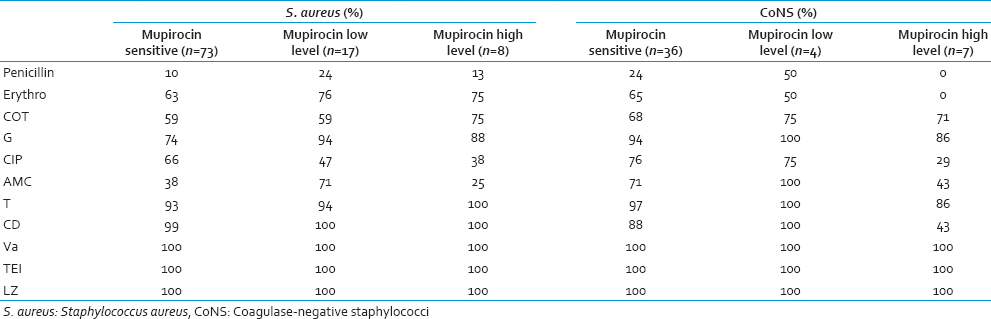
Among the 15 MupRH Staphylococcus spp., 5 showed iMLSB and 4 showed cMLSB [Table 3]. Occurrence of MupRH and MLSB [Figure 1] phenotype in same isolate may indicate carriage of both genes on same plasmids. The susceptibility of various antibiotics classes with mupirocin resistance is shown in Table 4.

DISCUSSION
Topical preparations of mupirocin first became available in 1985, since then it is widely used for management of infection and colonization of MRSA in both patients and medical personnel. The first report of mupirocin resistant S. aureus came shortly after its introduction (1987) from UK. In recent days, there is a worldwide increase in mupirocin resistance among S. aureus. Genetic basis of mupirocin resistance is defined. Low-level mupirocin resistance is due to point mutations in native isoleucyl-tRNA synthetase (IRS) gene (ileS). High-level resistance is due to a plasmid-mediated gene, mupA (ileS2), which encodes an additional modified IRS, which has less affinity for mupirocin. Recently, a novel mupB gene is also identified for high-level mupirocin resistance.[8]
The present study showed MupRH was more frequent in MSSA compared to MRSA (P < 0.05) and was more common among isolates from out-patients when compared to in-patients. This is attributed to the over-the-counter availability of mupirocin in community settings and more frequent usage in the general population for skin infections than for eradicating carriage or treating MRSA outbreaks in hospitals.[9] In our hospital, since last 5 years, mupirocin is the only topical preparation available for treatment of suspected staphylococcal infections. Our hospital utilized 20,000 mupirocin ointments (5 g) in 2013 accounting to the usage of 100 kg of mupirocin. The ointment is easily available over-the-counter from a private pharmacy. This increased usage of mupirocin has led to the development of resistance. It is interesting to note the widespread usage of mupirocin ointment for skin and soft tissue infection may be the reason for higher resistance rates among isolates obtained from pus samples. Higher prevalence of MupRL among S. aureus is clinically less significant as mupirocin ointment contains 20,000 mg/L (2%), which can effectively clear such strains.[4]
Abimanyu et al. from South India showed 32% MRSA ST239 were associated with MupRH and iMLSB.[10] The present study showed three isolates of MSSA associated with MupRH and iMLSB. The prevalence of MupRH in India among MRSA varied from 0% to 38.46% and among MSSA from 0% to 1.5%. Prevalence of mupirocin resistance among staphylococci from various parts of India has been tabulated and compared with the present study [Table 5]. Most of the studies are focused on MRSA than MSSA.

High-level mupirocin resistant S. aureus were more likely to be susceptible to cotrimoxazole, gentamycin and tetracycline and were more likely to be resistant to penicillin, ciprofloxacin and amoxicillin-clavulanic acid. Resistance to vancomycin, teicoplanin, and linezolid was not found. Hence, the judicial use of these antibiotics must be carefully employed.
The MupRH CoNS showed resistance to penicillin (100%), erythromycin (100%), ciprofloxacin (71%), clindamycin (57%) and amoxicillin-clavulanic acid (57%). The co-occurrence of mupirocin resistance and resistance to other antibiotics may indicate the organism carrying plasmid with multiple resistance genes. A higher rate of mupirocin resistance among MR-CoNS is a concern because; they can serve as a reservoir for horizontal transmission to S. aureus.[1718] Similar study by Teo et al. found mupirocin-resistant CoNS were concomitantly methicillin resistant.[19]
Cadilla et al. in their study found a strong association of HL mupirocin resistance with resistance to ≥ 4 non-β-lactam antimicrobial classes among S. aureus.[18] In the present study, association was found between MupHR and ciprofloxacin among S. aureus. Resistance in CoNS showed similar findings as that of Cadilla et al.[18]
Occurrence of methicillin resistance and mupirocin resistance in the same isolates is a cause for concern considering the role of mupirocin as a topical agent for MRSA elimination and may select the MRSA infection. Simor et al. in their study found the co-existence of high-level mupirocin resistance and fusidic acid resistance in same isolates.[20] Fusidic acid can be used both topically and orally. Use of fusidic acid for treatment of patients infected MupRH staphylococci can result in the selection of strains, which are multidrug resistant. As an alternative to topical antibiotic preparations, a hydrogen peroxide cream has been developed and used successfully.[21]
The present study correlates the prevalence of mupirocin resistance and its usage in clinical practice. Most of the previous studies have focused on MRSA outbreaks; very few define overall mupirocin resistance rates among Staphylococcus spp. Ours being a tertiary care hospital caters services to patients from the different geographical area. The information we report here may also apply to other hospitals in our city as well as in other cities where data are lacking. A Limitation of this study was the relatively small sample size.
CONCLUSION
The results of this study indicate that the rate of mupirocin resistance has been increased. Higher prevalence of mupirocin resistance was noticed among community-acquired than hospital-acquired strains. Continued surveillance for mupirocin resistance is important in order to retain the usefulness of this agent for the treatment and prevention of staphylococcal infections. Judicial prescription of mupirocin after knowing the susceptibility report should become standard practice by clinicians. Prolonged or widespread use of mupirocin in hospital or community must be stopped. Infection control and antibiotic policies have to be developed, audited, and reviewed regularly.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- The emergence of mupirocin resistance: A challenge to infection control and antibiotic prescribing practice. J Antimicrob Chemother. 1998;41:11-8.
- [Google Scholar]
- Interpretive criteria to differentiate low- and high-level mupirocin resistance in Staphylococcus aureus. J Med Microbiol. 2007;56(Pt 7):937-9.
- [Google Scholar]
- The prevalence of low- and high-level mupirocin resistance in staphylococci from 19 European hospitals. J Antimicrob Chemother. 1998;42:489-95.
- [Google Scholar]
- Five-test simple scheme for species-level identification of clinically significant coagulase-negative staphylococci. J Clin Microbiol. 2003;41:1219-24.
- [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). M100-S24. Performance Standards for Antimicrobial Susceptibility Testing: 24th Informational Supplement. Wayne, PA: CLSI; 2014.
- [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). M7-A9. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. (9th ed). Wayne, PA: CLSI; 2012.
- [Google Scholar]
- MupB, a new high-level mupirocin resistance mechanism in Staphylococcus aureus. Antimicrob Agents Chemother. 2012;56:1916-20.
- [Google Scholar]
- Emergence of high-level mupirocin resistance in coagulase-negative staphylococci associated with increased short-term mupirocin use. J Clin Microbiol. 2012;50:2947-50.
- [Google Scholar]
- Emergence of methicillin-resistant Staphylococcus aureus ST239 with high-level mupirocin and inducible clindamycin resistance in a tertiary care center in Chennai, South India. J Clin Microbiol. 2012;50:3412-3.
- [Google Scholar]
- Detection of methicillin and mupirocin resistance in Staphylococcus aureus isolates using conventional and molecular methods: A descriptive study from a burns unit with high prevalence of MRSA. J Clin Pathol. 2002;55:745-8.
- [Google Scholar]
- Mupirocin resistance in Staphylococcus aureus in an Indian hospital. Diagn Microbiol Infect Dis. 2007;58:125-7.
- [Google Scholar]
- Mupirocin resistance in clinical isolates of staphylococci in a tertiary care centre in South India. Indian J Med Microbiol. 2010;28:372-5.
- [Google Scholar]
- Prevalence of high and low level mupirocin resistance among staphylococcal isolates from skin infection in a tertiary care hospital. J Clin Diagn Res. 2013;7:238-42.
- [Google Scholar]
- Resistance pattern of mupirocin in methicillin-resistant Staphylococcus aureus in trauma patients and comparison between disc diffusion and E-test for better detection of resistance in low resource countries. J Lab Physicians. 2014;6:91-5.
- [Google Scholar]
- Prevalence of mupirocin resistant Staphylococcus aureus isolates among patients admitted to a tertiary care hospital. N Am J Med Sci. 2014;6:403-7.
- [Google Scholar]
- In vivo transfer of high-level mupirocin resistance from Staphylococcus epidermidis to methicillin-resistant Staphylococcus aureus associated with failure of mupirocin prophylaxis. J Antimicrob Chemother. 2005;56:1166-8.
- [Google Scholar]
- Association of high-level mupirocin resistance and multidrug-resistant methicillin-resistant Staphylococcus aureus at an academic center in the midwestern United States. J Clin Microbiol. 2011;49:95-100.
- [Google Scholar]
- High prevalence of mupirocin-resistant staphylococci in a dialysis unit where mupirocin and chlorhexidine are routinely used for prevention of catheter-related infections. J Med Microbiol. 2011;60(Pt 6):865-7.
- [Google Scholar]
- Mupirocin-resistant, methicillin-resistant Staphylococcus aureus strains in Canadian hospitals. Antimicrob Agents Chemother. 2007;51:3880-6.
- [Google Scholar]
- Mupirocin and Staphylococcus aureus: A recent paradigm of emerging antibiotic resistance. J Antimicrob Chemother. 2003;51:613-7.
- [Google Scholar]





