Translate this page into:
Proximal epithelioid sarcomatous dedifferentiation in secondary chondrosarcoma in a known case of multiple osteochondromatosis
Address for correspondence: Dr. Adarsh Barwad, Department of Pathology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry - 605 006, India. E-mail: publicationmail@gmail.com
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Osteochondroma is the most common benign bone tumor. Approximately 15% of osteochondromas occur as multiple lesions. Multiple osteochondromatosis has a higher risk of developing chondrosarcomas, which are of low grade with good prognosis. About 10% of all chondrosarcomas may undergo dedifferentiated change, which has a poorer prognosis. Dedifferentiated peripheral chondrosarcoma developing within an osteochondroma is extremely rare. Dedifferentiation usually occurs in the form of osteosarcoma, malignant fibrous histiocytoma, fibrosarcoma, or rhabdomyosarcoma. We report a case of proximal epithelioid sarcomatous dedifferentiation in secondary chondrosarcoma in a 39-year-old male with multiple osteochondromatosis in bilateral arm. To the best of our knowledge, epithelioid sarcomatous dedifferentiation has not been described in the literature.
Keywords
Osteochondroma
sarcoma
secondary
Introduction
Multiple osteochondromas (exostoses) hold a higher risk of developing chondrosarcomas.[123] Most secondary chondrosarcomas are of low grade and have a good prognosis.[14] Dedifferentiated chondrosarcomas, which have a poorer prognosis, can also arise in such a setting.[5] Dedifferentiation usually occurs in the form of malignant fibrous histiocytoma, osteosarcoma, fibrosarcoma, or rhabdomyosarcoma.[678] Epithelioid sarcomatous dedifferentiation is not mentioned in literature. We report a case of proximal epithelioid sarcomatous dedifferentiation in secondary chondrosarcoma in a 39-year-old male with multiple exostoses in bilateral arm.
Case Report
A 39-year-old male, with a history of multiple exostoses in bilateral arm, presented with an increasing mass in the right arm for 3 months. Contrast-enhanced computed tomography scan showed a heterogeneously enhancing mass arising from metaphyseal-diaphyseal region of proximal shaft of right humerus [Figure 1a].

- (a) Contrast-enhanced computed tomography showed a heterogeneously enhancing mass arising from metaphyseal-diaphyseal region of proximal shaft of right humerus. (b) Right-sided forequarter amputation gross specimen showed a mass measuring 28 cm × 27 cm × 20 cm in the right shoulder region
Fine-needle aspiration was attempted, which revealed many discrete, large, atypical, epithelial-like cells with eccentrically placed nucleus with evenly distributed chromatin, with moderate to abundant pale cytoplasm. Mitoses appeared to be increased. Overall picture was that of a malignant tumor with an epithelioid morphology. However, material was insufficient for immunocytochemistry.
Right-sided forequarter amputation was performed. The gross specimen received showed a large mass in the shoulder region, measuring 28 cm × 27 cm × 20 cm [Figure 1b]. Cut surface of the large mass had a variegated appearance, with both osseous and chondroid areas, admixed with focal areas of hemorrhages. The maximum cartilage thickness measured 4 cm. The tumor grossly showed cortical infiltration in the underlying humerus.
Microscopic examination of multiple sections from the tumor showed extensive chondroid differentiation with adjacent areas showing endochondral ossification and mineralization. The chondroid areas showed increase in cellularity, clustering of chondrocytes, and focal myxoid change, with focal atypia. Permeation into marrow spaces was noted [Figure 2a]. The sections from the adjacent reddish-brown soft-tissue areas showed abrupt transition into high-grade sarcomatous areas, wherein tumor cells were arranged in sheets and nodules with epithelioid differentiation [Figure 2b]. The cells exhibiting round to oval nuclei, coarse chromatin, conspicuous nucleoli, and moderate to abundant cytoplasm [Figure 2c]. Lymphovascular embolization was also seen.
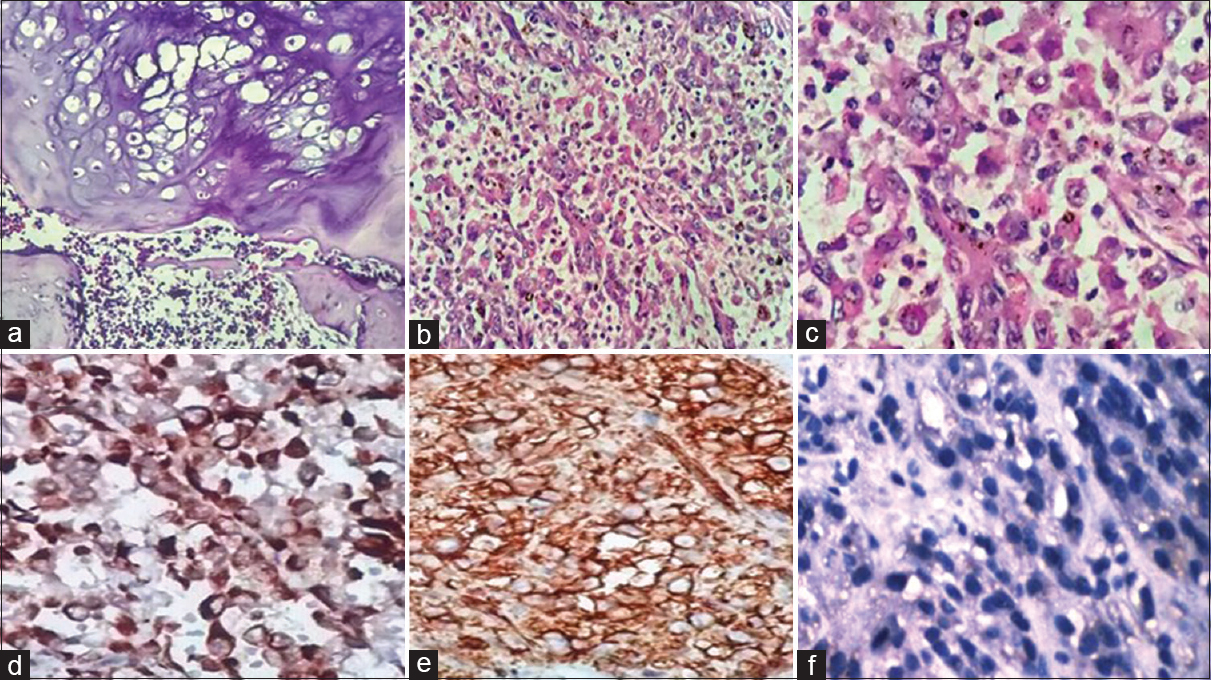
- (a) Histopathology showed low-grade chondrosarcoma permeating into marrow spaces (H and E, ×100). (b) High-grade sarcomatous areas composed of tumor cells arranged in sheets and nodules with epithelioid differentiation (H and E, ×100). (c) The tumor cells had round to oval nuclei, coarse chromatin, conspicuous nucleoli, and moderate to abundant cytoplasm (H and E, ×400). (d) Pancytokeratin positivity in the tumor cells in the sarcomatous area (immunohistochemistry, ×400). (e) Vimentin positivity in the tumor cells in the sarcomatous area (immunohistochemistry, ×400). (f) Loss of integrase interactor-1 in the sarcomatous area (immunohistochemistry, ×400)
On immunohistochemistry (IHC), the tumor cells in the sarcomatous areas were positive for pancytokeratin, epithelial membrane antigen (EMA), cytokeratin 20, CD99, vimentin, and CD34 [Figure 2d and e]. The tumor cells were negative for integrase interactor-1 (INI1), desmin, S-100, Bcl-2, CD68, smooth muscle actin (SMA), MyoD1, and CD31 [Figure 2f].
Based on the clinicoradiological findings, gross features, and histomorphologic features, an impression of proximal epithelioid sarcomatous dedifferentiation in secondary chondrosarcoma in a known case of multiple osteochondromatosis was made.
The patient was discharged on the 12th postoperative day and advised to visit the orthopedics outpatient department for follow-up. However, the patient never turned up and was lost to follow-up.
Discussion
Osteochondroma is the most common benign bone tumor (more than 30% of all benign bone tumors) and usually occurs in the metaphyseal region of the long bones.[1] It is a cartilage-capped bony outgrowth on the surface of the bone containing a marrow cavity that is continuous with that of the underlying bone.[12] The vast majority (85%) of osteochondromas present as solitary, nonhereditary lesions.[1] However, approximately 15% of osteochondromas occur as multiple lesions.[12] A diagnosis of multiple osteochondromatosis is made when at least two osteochondromas of the juxta-epiphyseal region of long bones are observed radiologically.[2] Approximately 62% of the patients with multiple osteochondromas have a positive family history, and/or mutation in one of the EXT (EXT1 and EXT2) genes can be detected.[12]
The most severe complication of osteochondroma is malignant transformation, which is estimated to occur in 0.5%–5% of patients.[23] Malignant transformation leads to a secondary peripheral chondrosarcoma in 94% of the cases and rarely to a secondary osteosarcoma or spindle cell sarcoma.[123] The risk is substantially higher in patients with multiple osteochondromatosis (5%–25%) as compared to solitary ones (1%–2%).[1] Development of a secondary chondrosarcoma in a case of osteochondroma is suspected if there is a persistence of growth of the tumor after puberty, the presence or increase in pain, or a thickness over 1 cm of the cartilaginous cap in adults.[2] The average age of the secondary chondrosarcoma patient is 35 years, younger than those with primary tumors.[134] It has male preponderance, and flat bones such as pelvic bones are most commonly affected.[14] Most secondary peripheral chondrosarcomas are of low grade and generally carry a good prognosis.[14] In peripheral chondrosarcoma, EXT1 and/or EXT2 genes are affected on the genomic level, by mutation and/or deletions.[5]
Dedifferentiated chondrosarcoma is a distinct form of chondrosarcoma where a high-grade sarcoma is found adjacent to a low-grade chondrosarcoma, and about 10% of all chondrosarcomas may undergo dedifferentiated change.[5] It has a poor prognosis with a 5-year survival rate of 0%–20% approximately.[23456] Dedifferentiated peripheral chondrosarcoma developing within an osteochondroma is extremely rare.[2367] Dedifferentiation usually occurs in the form of osteosarcoma, malignant fibrous histiocytoma, fibrosarcoma, or rhabdomyosarcoma.[78] To the best of our knowledge, epithelioid sarcomatous dedifferentiation is not described in the literature.
Proximal epithelioid sarcoma is a rare variant of epithelioid sarcoma presenting in proximal body sites with a more aggressive behavior.[9] Histologically distinctive features are prominence of the epithelioid cell component in a multinodular pattern or a sheet-like growth pattern. A granuloma-like pattern has been described.[910] The tumor cells are large cells with vesicular nuclei and prominent nucleoli and have a frequent rhabdoid appearance. Mitotic images and areas of necrosis are often present.[910] Proximal-type epithelioid sarcoma and the classic form of epithelioid sarcoma have a similar immunohistochemical staining profile. These lesions are characteristically positive with cytokeratins, vimentin, and EMA and exhibit loss of INI1 staining.[10] INI1 (SMARCB1), located on chromosome 22q11.2, is inactivated in epithelioid sarcomas.[10]
In the present case, a panel of IHC markers was done to confirm it as epithelioid sarcomatous component. A panel of negative IHC markers was done such as S-100 to rule out epithelioid malignant peripheral nerve sheath tumor, CD31 to rule out epithelioid angiosarcoma, SMA to rule out epithelioid leiomyosarcoma, desmin and MyoD1 to rule out rhabdomyosarcoma, and Bcl-2 to rule out synovial sarcoma.
In multiple osteochondromatosis and in peripheral chondrosarcomas, EXT1 and/or EXT2 genes undergo mutation and/or deletions leading to decreased expression.[25] EXT1 and EXT2 are located at 8q24 and 11p11-p12, respectively.[2] Mutations and/or deletions of EXT1 and/or EXT2 lead to aberrant heparan sulfate proteoglycans (HSPG) synthesis.[5] HSPGs including CD44 and CD44 bearing variable exon 3 (CD44v3) are involved in signal transduction of several pathways, including the Indian hedgehog protein (IHH), transforming growth factor beta, and fibroblastic growth factor (FGF) signaling.[25] Parathyroid hormone-like hormone (PTHLH) is a downstream signaling of IHH pathway.[2] Responsible for chondrocyte proliferation, it is absent in osteochondroma and is being upregulated upon malignant transformation of osteochondroma.[2] Thus, expression of PTHLH is increased in secondary peripheral chondrosarcoma and is low or absent in osteochondroma.[5] As it undergoes dedifferentiation, expression of PTHLH decreases in the chondrogenic component and so is the expression of CD44v3.[5] Whereas FGF signaling and CD44 expression are increased in the dedifferentiated chondrogenic component.[5] These alterations suggest that these changes maybe of importance in the transformation of multiple osteochondromatosis to chondrosarcoma and then toward dedifferentiated chondrosarcoma. However, the exact role of these pathways needs to be further elucidated.
As such dedifferentiated component in a secondary chondrosarcoma may be easily overlooked clinically and radiographically. Therefore, adequate sampling and careful histologic analysis of all cartilage lesions of bone surface are essential to prevent underreporting of high-grade sarcomatous dedifferentiation foci.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Osteochondromas: Review of the clinical, radiological and pathological features. In Vivo. 2008;22:633-46.
- [Google Scholar]
- Malignant transformation of a multiple cartilaginous exostosis – A case report. Int Orthop. 1997;21:133-6.
- [Google Scholar]
- Secondary chondrosarcoma in osteochondroma: Report of 107 patients. Clin Orthop Relat Res. 2003;411:193-206.
- [Google Scholar]
- Dedifferentiated peripheral chondrosarcomas: Regulation of EXT-downstream molecules and differentiation-related genes. Mod Pathol. 2009;22:1489-98.
- [Google Scholar]
- Dedifferentiated peripheral chondrosarcomas. A report of seven cases. Cancer. 1989;63:2054-9.
- [Google Scholar]
- Dedifferentiated chondrosarcomas arising in preexisting osteochondromas. J Bone Joint Surg Am. 2007;89:987-93.
- [Google Scholar]
- Dedifferentiated chondrosarcoma. A study of 13 clinical cases and review of the literature. Rev Chir Orthop Reparatrice Appar Mot. 1994;80:669-80.
- [Google Scholar]
- Proximal-type epithelioid sarcoma: A clinicopathologic study of 20 cases. Mod Pathol. 2001;14:655-63.
- [Google Scholar]





