Translate this page into:
Rare mold infections from a tertiary care institute in South India: A case series
*Corresponding author: Sukanya Sudhaharan, Associate Professor, Department of Microbiology, Nizams Institute of Medical Sciences, Hyderabad, Telangana, India. sukanyavimala@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sudhaharan S, Pamidimukkala U, Verma N, Katkam S. Rare mold infections from a tertiary care institute in South India – A case series. J Lab Physicians. 2024;16:552-5. doi: 10.25259/JLP_80_2024
Abstract
With the increasing incidence of immunocompromised patients, invasive infections caused by rare molds are increasing. Here, we report four cases of rare mold infections in immunocompromised and immunocompetent patients. The first case was Scedosporium apiospermum infection in a road traffic accident, the second and third cases were subcutaneous infection by Medicopsis romeroi in post-renal transplant patients, and the fourth case was subcutaneous infection by Phaeoacremonium parasiticum. Invasive fungal infections caused by emerging fungi are on the rise in both immunocompromised and immunocompetent patients. Microbiologists and clinicians should be aware of these rare fungal infections and not consider them contaminants since early identification and appropriate management would help to prevent the mortality associated with these infections.
Keywords
Scedosporium apiospermum
Medicopsis romeroi
Phaeoacremonium parasiticum
INTRODUCTION
With the increasing incidence of immunocompromised patients, invasive infections caused by fungal pathogens are on the rise.[1] Aspergillus was the common cause of invasive mold infection.[2] In recent decades, infections due to rare molds have been reported. This may be due to the use of prolonged antifungal prophylaxis in immunocompromised patients, an increase in the survival rate of patients, and increased immunosuppression.[3] Moreover, newer molecular diagnostic methods have helped in the diagnosis of these infections, which are difficult to identify by conventional methods.[4] Here, we report four cases of rare mold infections in immunocompromised and immunocompetent patients. This was a retrospective study from January 2019 to December 2023 (5 years). The mold infections other than Aspergillus, Mucormycetes, and Fusarium spp. were included in the study. Case records collected the complete clinical history and management of the patients.
Microbiological processing
Direct microscopy of the samples was done by potassium hydroxide-Calcofluor white stain. The samples were inoculated on Sabouraud dextrose agar with chloramphenicol and incubated at 25°C. Sample processing was done in Biosafety Cabinet Class II (A2). Growth was observed after 48–72 h of incubation. The isolates were identified and confirmed by slide culture/sequencing.
CASE SERIES
Case 1
A 13-year-old boy who had a fracture of the left lower tibia following a road traffic accident was admitted to the hospital. He was treated with debridement, external fixation, and primary reconstruction using a free flap. Empirical antibiotics with amikacin and piperacillin-tazobactam were started. After 3 days, there was a flap failure. The flap was removed, and the tissue under the flap was necrotized. The tissue was debrided and sent to microbiological processing.
Direct microscopy of the sample revealed branching septate hyphae.
After 48 h of incubation, the culture showed a grayish-white colony with a grayish-black reverse. The slide culture of the colony showed numerous single-celled, pale brown, broadly clavate to ovoid conidia, rounded above with a truncate base, which was identified as Scedosporium apiospermum [Figure 1].
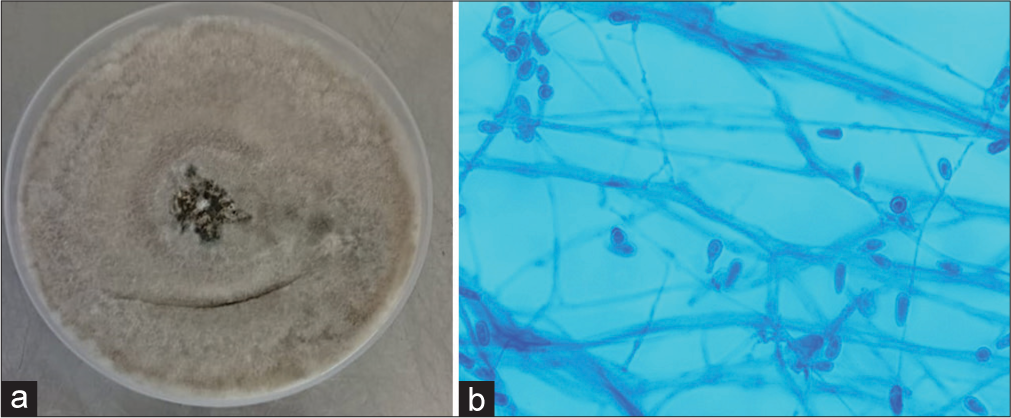
- (a) Culture of Scedosporium apiospermum on Sabouraud dextrose agar. (b) Lactophenol cotton blue mount of S. apiospermum ×400 magnification.
After the culture report, the patient’s limb was amputated, and he was started on oral therapy with voriconazole. The patient’s condition improved, and he was discharged in a stable condition with follow-up advice.
Case 2
A 50-year-old patient underwent a renal transplant in 2020 and was on triple immunosuppression (tacrolimus 5 mg, mycophenolate mofetil 1.5 g, and wysolone 20 mg). After 2 years and 6 months (July 2022), he came with complaints of swelling in the right leg for 1 month. Incision and drainage of the abscess were done, and the pus was sent to microbiology.
Direct microscopy of the samples revealed pigmented septate hyphae. The culture showed growth of gray to black colony after 72 h of incubation. The culture was non-sporulating when examined microscopically with lactophenol cotton blue mount. DNA sequencing of the ITS4 and ITS5 regions identified the mold as Medicopsis romeroi [Figure 2].

- Culture of Medicopsis romeroi on Sabouraud dextrose agar.
The patient was treated with voriconazole 200 mg twice daily for 6 months. There was no relapse on follow-up.
Case 3
A 55-year-old female patient, 1½-year post-renal transplant and on immunosuppression (tacrolimus 4 mg, mycophenolate mofetil 1.5 g, and wysolone 20 mg) came with complaints of painless swelling of the left proximal interphalangeal joint of the little finger for 3 months which was increasing in size. Excision biopsy of the lesion was done, and the sample was sent to microbiology. The patient was treated with voriconazole 200 mg twice daily for a year. There was no relapse on follow-up.
Direct microscopy of the samples revealed pigmented septate hyphae. The culture showed growth of a gray-to-black colony after 72 h of incubation. The culture was non-sporulating when examined microscopically with lactophenol cotton blue mount. DNA sequencing of the ITS4 and ITS5 regions identified the mold as M. romeroi [Figure 2].
Case 4
A 57-year-old male, a known hypertensive for 10 years, occupation being agriculture, came with complaints of a cyst on the left index finger for 10 days. He gave a history of trauma on the finger. An excision biopsy of the cyst was done. Direct microscopy of the sample revealed pigmented septate hyphae.
Culture showed growth of a whitish-gray colony after 72 h of incubation. Microscopy by lactophenol cotton blue mount revealed brownish hyphae with thick-walled brown cylindrical conidia with small, funnel-shaped collarettes. Thin-walled, cylindrical conidia are seen, confirming it as Phaeoacremonium parasiticum [Figure 3] morphologically. The culture was confirmed by DNA sequencing of the ITS4 and ITS5 regions.
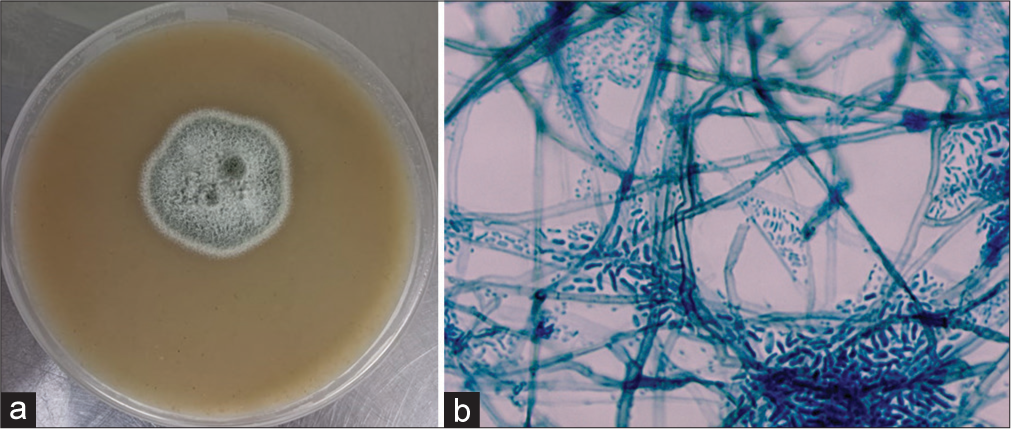
- (a) Culture of Phaeoacremonium parasiticum on Sabouraud dextrose agar. (b) Lactophenol cotton blue mount of P. parasiticum ×400 magnification.
The patient was treated with voriconazole 200 mg twice daily for 2 months with advice to follow up.
DISCUSSION
Fungal infections are one of the leading causes of morbidity and mortality, particularly in immunocompromised patients.[5] The epidemiology of mold infections has changed over the decades and there has been a rise in rare mold infections due to the increased use of corticosteroids, immunosuppressants, antineoplastics, broad-spectrum antibiotics and antifungals.[4]
In the first case, S. apiospermum was isolated from tissue in a road traffic accident, which was not reported in the literature. The fungus is present in the environment, such as soil and sewage, and can affect both immunocompromised and immunocompetent hosts. It is an opportunistic pathogen causing a wide range of infections, from superficial and systemic to disseminated infections.[6] It can affect paranasal sinuses, lungs, skin, soft tissue, central nervous system, and bones.[7] S. apiospremum is resistant to amphotericin B and has variable susceptibility to azoles. Voriconazole has good in vitro activity and is the first line of treatment along with surgery in localized infections.[8] Our patient was successfully treated with voriconazole with debridement.
In the second and third cases, M. romeroi was isolated from subcutaneous infection in renal transplant patients. It is a dematiaceous fungus that was previously known as Pyrenochaeta romeroi. The fungus is an environmental pathogen present in soil and plants of subtropical and tropical regions. It was a rare human pathogen, but recently, it was known to cause infection in immunocompromised patients, especially in solid organ transplants. These patients usually have a history of traumatic inoculation.[9] There were about 52 cases of M. romeroi reported in the literature; the majority (38/52) of the patients had subcutaneous infections, and 63% of other patients were immunosuppressed. A combination of surgery and antifungal therapy would be helpful in remission. Antifungal therapy includes voriconazole, itraconazole, and amphotericin B.[10] Our patients were treated with voriconazole. In the fourth case, P. parasiticum was isolated from a subcutaneous infection. It is an agent of opportunistic phaeohyphomycosis and is mainly found in the environment and is considered a plant pathogen. The spectrum of disease varies from subcutaneous infections to disseminated disease with multiorgan involvement.[11] The route of entry may be due to traumatic inoculation, and the agricultural workers are at risk.[12] Treatment includes a combination of surgery with amphotericin B/azoles.[11] In the present case, the patient was cured with the removal of the cyst along with voriconazole therapy.
The present series highlights the infections caused by environmental fungus in immunocompetent (cases 1 and 4) and immunocompromised patients (cases 2 and 3). The route of entry was traumatic inoculation in cases 1 and 4, and though the patients in cases 2 and 3 did not give a history, there would be a possibility of traumatic inoculation. The isolates were slow-growing and were difficult to identify by conventional methods. Molecular methods such as DNA sequencing could confirm the identification. Treatment for all the patients was a combination of surgery and antifungal therapy. In the present series, the infections were localized, and the patients responded to surgery with voriconazole therapy. The patients were discharged in a stable condition.
CONCLUSIONS
Invasive fungal infections caused by environmental fungi are on the rise in both immunocompromised and immunocompetent patients.
Microbiologists and clinicians should be aware of these rare fungal infections and should not consider them contaminants since early identification and appropriate management would help prevent the morbidity and mortality associated with them.
Ethical approval
The research/study was approved by the Institutional Review Board at Nizam’s Institute of Medical Sciences, number 3038, dated 2023.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Rare mould infections caused by Mucorales, Lomentospora prolificans and Fusarium in San Diego, CA: The role of antifungal combination therapy. Int J Antimicrob Agents. 2018;52:706-12.
- [CrossRef] [PubMed] [Google Scholar]
- Non-Aspergillus mould lung infections. Eur Respir Rev. 2022;31:220104.
- [CrossRef] [PubMed] [Google Scholar]
- Changing epidemiology of rare mould infections: Implications for therapy. Drugs. 2007;67:1803-12.
- [CrossRef] [PubMed] [Google Scholar]
- Rare fungal infectious agents: A lurking enemy. F1000Res. 2017;6:1917.
- [CrossRef] [PubMed] [Google Scholar]
- Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi (Basel). 2017;3:57.
- [CrossRef] [PubMed] [Google Scholar]
- A case of invasive fungal infection due to Scedosporium apiospermum in a patient with psoriasis. Infect Drug Resist. 2023;16:5085-90.
- [CrossRef] [PubMed] [Google Scholar]
- Scedosporium apiospermum infections and the role of combination antifungal therapy and GM-CSF: A case report and review of the literature. Med Mycol Case Rep. 2016;11:40-3.
- [CrossRef] [PubMed] [Google Scholar]
- ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. Clin Microbiol Infect. 2014;20:27-46.
- [CrossRef] [PubMed] [Google Scholar]
- Medicopsis romeroi nodular subcutaneous infection in a kidney transplant recipient. Int J Infect Dis. 2020;95:262-4.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrent subcutaneous phaeohyphomycosis due to Medicopsis romeroi A case report in a Dermatomyositis patient and review of the literature. Microorganisms. 2022;11:3.
- [CrossRef] [PubMed] [Google Scholar]
- Phaeoacremonium parasiticum infections confirmed by beta-tubulin sequence analysis of case isolates. J Clin Microbiol. 2006;44:2207-11.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous infection by Phaeoacremonium parasiticum. Rev Iberoam Micol. 2019;36:90-2.
- [CrossRef] [PubMed] [Google Scholar]






