Translate this page into:
Relationship Between Insulin Resistance and Mean Platelet Volume in Gestational Diabetes Mellitus
Address for correspondence: Dr. Suleyman Baldane, E-mail: baldane42@hotmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives:
A few studies have investigated the relationship between mean platelet volume (MPV) and gestational diabetes mellitus (GDM), and in these studies the relationship between MPV and insulin resistance has not been analyzed. Our aim in this study was to compare MPV values of the pregnant women with or without GDM and evaluate the relationship between MPV and homeostasis model assessment insulin resistance index (HOMA-IR) in pregnant women.
Materials and Methods:
One hundred and fourteen with GDM measurements being obtained before any dietary advice or therapy with insulin or hypoglycemic agents were given, and 76 with healthy pregnant women were included the study.
Results:
In the group with GDM, MPV value was found to be significantly higher than that of the control group (10.2 fl [8.0–12.2] vs. 9.9 fl [5.81–10.9], P = 0.004). HOMA-IR value was detected to be significantly higher in the group with GDM (2.46 [1.5–5.88] vs. 1.30 [0.17–2.92], P < 0.001). A positive correlation between MPV and HOMA-IR was found (r = 0.30, P = 0.002).
Conclusion:
We have shown that MPV was significantly elevated in GDM patients when compared to healthy pregnant women. Furthermore, we found that there was a positive correlation between MPV and HOMA-IR.
Keywords
Gestational diabetes mellitus
insulin resistance
mean platelet volume
INTRODUCTION
Gestational diabetes mellitus (GDM) is defined as the first onset of glucose intolerance in pregnancy and is seen in 3–5% of all pregnancies.[1] Pregnancy is a process associated with insulin resistance and becomes prominent especially during the last two trimesters. Increases in cortisol, prolactin, human placental lactogen, and leptin levels play an important role in the insulin resistance seen during pregnancy.[2] In pregnancy, the most important adaptation mechanism effective in the regulation of normal glucose metabolism is a functional enhancement of pancreatic beta cells, and in cases with inadequate adaptation, hyperglycemia develops.[3] In most patients with GDM, glucose intolerance resolved during the postnatal period; however these patients have a lifetime risk of type 2 diabetes and cardiovascular disease.[45]
Platelet volume is a marker of platelet activation and function that can be measured simply and relatively cost effectively using routine hemogram devices that express this parameter as mean platelet volume (MPV).[6] Larger platelets are metabolically and enzymatically more active.[7] An increase in the MPV value has been demonstrated in conditions closely associated with insulin resistance including metabolic syndrome, obesity, impaired fasting glucose, diabetes mellitus, and hypertension.[891011]
Several studies have investigated the relationship between MPV and GDM; however, the relationship between MPV and insulin resistance has not been analyzed. Our aim in the present study was to compare MPV values of pregnant women with or without GDM and evaluate the relationship between MPV and the homeostasis model assessment insulin resistance index (HOMA-IR) in pregnant women.
MATERIALS AND METHODS
The present study was a cross-sectional, case–control study. The design of the present study was approved by the Ethical Committee and Institutional Review Board of Selcuk University Faculty of Medicine where the study was conducted. Written informed consents were obtained from all participants.
All recruited pregnant women were selected randomly from women admitted to our gynecology and endocrinology outpatient clinic between January 2013 and May 2014. The study was not blinded, and randomization was generated by a third physician using tables of random numbers. Age, gestational age, systolic blood pressure (SBP), and diastolic blood pressure (DBP), smoking status were noted for all participants. Prepregnancy body mass index (BMI) was calculated prior to pregnancy as the ratio of weight divided by height squared (kg/m2). Criteria for exclusion was pregestational diabetes, preeclampsia, eclampsia, pregnancy-induced hypertension, history of hypertension before pregnancy, taking anticoagulation medicine, myeloproliferative disorders, malignancy, chronic inflammatory disease, and acute or chronic infection.
All women screened for GDM with 50 g glucose challenge test. Women with a 1 h glucose level of 140 mg/dl or more proceeded to 100 g oral glucose tolerance test (OGTT) and using Carpenter and Coustan guidelines,[12] women with two or more abnormal values were diagnosed as having GDM.
One hundred and fourteen with GDM with measurements being obtained before any dietary advice or therapy with insulin or hypoglycemic agents was given, and 76 with healthy pregnant women were included the study.
For the serum analysis, samples were obtained after overnight fasting and at the time of OGTT. All blood samples were determined (as a part of complete blood count) using an Automated Hematology Analyzer Cell-Dyn 3200 (Abbott Diagnostics, Abbot Park, IL, USA) for measurement of MPV. In our clinic, the MPV reference range is determined as 7–11 fl. Lipid parameters (total cholesterol, triglyceride, high-density lipoprotein (HDL), and insulin were measured by standardized methods using auto-analyzers. The low-density lipoprotein (LDL) cholesterol levels were calculated according to the formula described by Friedewald et al.[13] HOMA-IR was calculated as follows:[14] ([fasting insulin, mIU/ml] × [fasting glucose, mg/dl]/405).
Statistical analyses were performed using the SPSS software version 16 (SPSS Inc., Chicago, IL). Continuous data were presented as means ± standard deviation or medians (minimum-maximum), as appropriate. Proportions were compared using the Chi-square test or Fisher's exact test, when applicable. The level of significance was determined using the student t-test for normally distributed values, and the Mann–Whitney U-test was used for non-normally distributed values. A P < 0.05 was considered statistically significant. Spearman's rank correlation analysis was used to assess the association between MPV and HOMA-IR and insulin.
RESULTS
Clinical and laboratory characteristics of the patients in the GDM and control groups are presented in Table 1. A significant difference was not observed regarding age, prepregnancy BMI, smoking, SBP and DBPs, gestational age, total cholesterol, LDL, HDL, and platelet values for each group [Table 1]. In the GDM group fasting glucose, insulin, and triglyceride levels were found to be significantly higher than control group [Table 1].
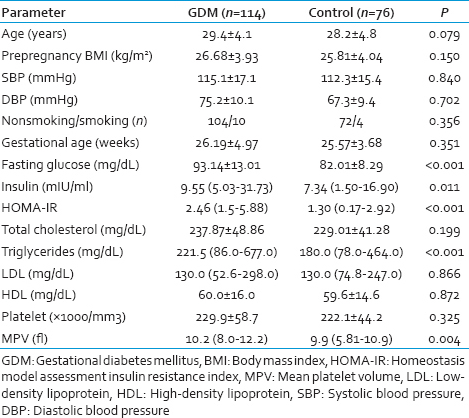
In the group, with GDM MPV and HOMA-IR values were also significantly higher than those of the control group (10.2 fl [8.0–12.2] vs. 9.9 fl [5.81–10.9], P = 0.004 and 2.46 [1.5–5.88] vs. 1.30 [0.17–2.92], P < 0.001, respectively).
A positive correlation between MPV and insulin and HOMA-IR was found (r = 0.30, P = 0.002 and r = 0.235 P = 0.008, respectively) [Figure 1].
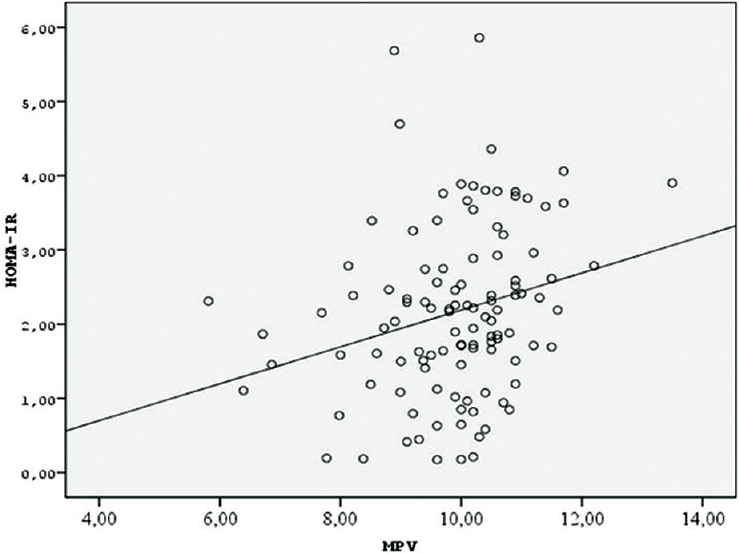
- Correlation between homeostasis model assessment insulin resistance index and mean platelet volume
DISCUSSION
In this study, where gestational diabetic pregnancies were compared to normal pregnancies, the patients with GDM were observed to have a higher MPV. Furthermore, the positive correlation between MPV and HOMA-IR was demonstrated, which is the first study the correlation between insulin resistance and MPV has been investigated.
Evidence concerning the presence of a positive correlation between MPV and type 2 diabetes was first reported by Sharpe and Trinick[15] who noted a significant increase in MPV values in diabetic patients compared with nondiabetic patients (8.9 vs. 8.0 fl, P < 0.001). A later study by Hekimsoy et al. on 145 type 1 diabetic and 100 nondiabetic patients also showed significantly higher MPV values in the diabetic group (10.6 versus 9.1 fl, P < 0.001).[9] Similarly, in two small-scale case-controlled studies, significantly increased MPV values of diabetic patients was reported when compared with nondiabetic patients.[1011] In their study, encompassing groups with diabetes, impaired fasting glucose subjects, and healthy control subjects. Coban et al. demonstrated a positive correlation between MPV and the severity of hyperglycemia.[11] In a recent study, consisting of 13,021 diabetic patients, a positive correlation among MPV, fasting glucose, and HbA1c (all P < 0.001); and a significant correlation between MPV and severity of diabetes were reported.[16]
The underlying mechanism producing higher MPV values in diabetic patients have not been fully elucidated. One of the suggested mechanisms is osmotic swelling in platelets induced by hyperglycemia.[17] Another potential mechanism is that insulin triggers the production of platelets larger than megakaryocytes.[18] As an alternative view, MPV values might reflect a higher platelet turnover rate and increased production of young platelets.[19]
Pathogenesis of type 2 diabetes and GDM is nearly similar, and the fundamental underlying basis of the investigation of the relationship between type 2 diabetes and pathogenesis of GDM is morphological changes in platelets, which have been demonstrated in type 2 diabetic patients. Especially in the last two trimesters of pregnancy, markedly conspicuous insulin resistance accompanies pregnancy. In a large proportion of the patients with GDM, insulin resistance resolves during the postnatal period. The main underlying mechanism of the alterations in platelet morphology and functions in patients with GDM might be insulin resistance and the related development of hyperglycemia, as seen in type 2 diabetes. A few studies have investigated the association between MPV and GDM. Bozkurt et al. reported significantly increased MPV values in patients with GDM relative to the control group,[20] and Erikçi et al. reinforced the findings of Bozkurt et al.[21] In contrast, in a cross-sectional study, similar levels of MPV were found in healthy pregnant women and those with GDM,[22] and higher levels of a platelet adhesion molecule (CD62P [P-selectin]) were detected in the group with GDM when compared with the control group. In a recent study, Iyidir et al. demonstrated the presence of significantly higher MPV values in the group with GDM relative to the control group and also indicated significantly decreased MPV values in the group with GDM secondary to the resolution of insulin resistance during the postnatal period.[23] The authors also reported significantly higher MPV values in patients with GDM, who received insulin treatment when compared with those on diet therapy.[23] The outcomes of the study performed by Iyidir et al. demonstrated a correlation between increased MPV values and insulin resistance in pregnant women, and their results are in accordance with our findings that demonstrated a positive correlation between MPV values and HOMA-IR levels.
Two studies investigated the correlation between MPV and insulin resistance over HOMA-IR. Varol et al. divided 72 nonobese nondiabetic patients into two groups based on HOMA-IR values and found significantly higher MPV values in the group with insulin resistance.[24] They also detected a positive correlation between MPV and HOMA-IR levels (r = 0.30, P = 0.054).[24] In a more recent study, Elsherbiny et al. divided 60 patients with coronary slow-flow phenomenon into two groups based on HOMA-IR values and detected significantly higher MPV values[25] and a positive correlation between MPV and HOMA-IR values (r = 0.52, P < 0.001).[25]
Previous studies of the relationship between GDM and MPV did not document a correlation between MPV and insulin resistance. In our study, we detected a positive correlation between MPV and HOMA-IR values (r = 0.30, P = 0.002). We speculate that there may be a close relationship between insulin resistance and MPV, which is an indicator of platelet activation in patients with GDM.
There are several limitations to our study. This was an observational single-institution study that had a relatively small sample size. We could not gain access to treatment modality, posttreatment information, and postnatal MPV values; and we were unable to analyze perinatal outcomes.
CONCLUSION
MPV can be a potential marker in the identification of patients with GDM. We demonstrated a positive correlation between MPV and insulin resistance. The possible effect of this correlation on perinatal outcomes and its role in the selection of treatment modality for GDM should be clarified. Further prospective studies should be performed with the participation of more individuals.
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- The epidemiology of diabetes and pregnancy in the U.S 1988. Diabetes Care. 1995;18:1029-33.
- [Google Scholar]
- The relationship between adipose tissue-derived hormones and gestational diabetes mellitus (GDM) Endokrynol Pol. 2014;65:134-42.
- [Google Scholar]
- Glucose tolerance status in pregnancy: A window to the future risk of diabetes and cardiovascular disease in young women. Curr Diabetes Rev. 2009;5:239-44.
- [Google Scholar]
- Gestational diabetes mellitus: The prevalence of glucose intolerance and diabetes mellitus in the first two months post partum. Am J Obstet Gynecol. 1990;163:93-8.
- [Google Scholar]
- Are platelet volume indices of clinical use? A multidisciplinary review. Ann Med. 2012;44:805-16.
- [Google Scholar]
- Mean platelet volume as a predictor of cardiovascular risk: A systematic review and meta-analysis. J Thromb Haemost. 2010;8:148-56.
- [Google Scholar]
- The mean platelet volume in patients with obesity. Int J Clin Pract. 2005;59:981-2.
- [Google Scholar]
- Mean platelet volume in type 2 diabetic patients. J Diabetes Complications. 2004;18:173-6.
- [Google Scholar]
- Mean platelet volume in patients with type 2 diabetes mellitus. Platelets. 2004;15:475-8.
- [Google Scholar]
- The mean platelet volume in subjects with impaired fasting glucose. Platelets. 2006;17:67-9.
- [Google Scholar]
- Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499-502.
- [Google Scholar]
- The relationship between diabetes, metabolic syndrome, and platelet activity as measured by mean platelet volume: The National Health And Nutrition Examination Survey, 1999-2004. Diabetes Care. 2012;35:1074-8.
- [Google Scholar]
- Effects of sorbinil treatment on erythrocytes and platelets of persons with diabetes. Diabetes Care. 1986;9:36-9.
- [Google Scholar]
- Effect of insulin on murine megakaryocytopoiesis in a liquid culture system. Cell Struct Funct. 1987;12:311-6.
- [Google Scholar]
- Role of reticulated platelets and platelet size heterogeneity on platelet activity after dual antiplatelet therapy with aspirin and clopidogrel in patients with stable coronary artery disease. J Am Coll Cardiol. 2008;52:743-9.
- [Google Scholar]
- The mean platelet volume in gestational diabetes. J Thromb Thrombolysis. 2006;22:51-4.
- [Google Scholar]
- Could mean platelet volume be a predictive marker for gestational diabetes mellitus? Hematology. 2008;13:46-8.
- [Google Scholar]
- Study on the variation of platelet function in pregnancy induced hypertension and gestational diabetes mellitus. Zhonghua Fu Chan Ke Za Zhi. 2005;40:25-8.
- [Google Scholar]
- Elevated mean platelet volume is associated with gestational diabetes mellitus. Gynecol Endocrinol. 2014;30:640-3.
- [Google Scholar]
- Mean platelet volume is associated with insulin resistance in non-obese, non-diabetic patients with coronary artery disease. J Cardiol. 2010;56:154-8.
- [Google Scholar]
- Mean platelet volume and its relation to insulin resistance in non-diabetic patients with slow coronary flow. J Cardiol. 2012;59:176-81.
- [Google Scholar]





