Translate this page into:
Role of immature platelet fraction in etiological diagnosis of thrombocytopenia
*Corresponding author: Vijay Kumar, Department of Pathology, Atal Bihari Vajpayee Institute of Medical Sciences and Dr Ram Manohar Lohia Hospital, New Delhi, India. vijaypgi1@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Bajaj H, Rajpal T, Sharma M, Singh P, Hemal A, Kumar V. Role of immature platelet fraction in etiological diagnosis of thrombocytopenia. J Lab Physicians. 2024;16:496-500. doi: 10.25259/JLP_107_2024
Abstract
Objectives:
Immature platelet fraction (IPF) is a newer automated parameter that measures the ratio of reticulated platelets to a total number of platelets. A measure of reticulated platelets determines the rate of thrombopoiesis which can help in differential diagnosis of thrombocytopenia. The study aims to evaluate the relationship between IPF and causes of thrombocytopenia and establish its clinical utility.
Materials and Methods:
The study was a prospective observational study conducted for 9 months. A total of 70 cases with an equal number of healthy age-matched controls were included in the study. Based on the pathogenesis of thrombocytopenia, the cases were grouped into platelet hypoproduction, hyperdestruction, and megaloblastic anemia. The association between IPF values among control and different case groups was evaluated.
Statistical analysis:
Assuming a 95% confidence level, the sample size calculated is 61 subjects. Based on the etiopathogenesis of thrombocytopenia, cases were categorized into three groups. Qualitative variables were compared using the Chi-square test/Fisher’s exact test. Quantitative variables were compared using unpaired t-test/Mann–Whitney test. P < 0.05 was considered significant at a 95% confidence level.
Results:
The reference range of IPF among healthy controls was estimated to be 0.6–6.8%. The mean IPF was significantly higher in the hyperdestructive group (10.6%) as compared to the hypoproductive group (3.6%). The optimal cutoff value of IPF for differentiating hyperdestruction causes from hypoproduction causes was 8.20% with a sensitivity of 75% and specificity of 87.5%.
Conclusions:
IPF can be used as an initial tool in the diagnostic evaluation of thrombocytopenia.
Keywords
Thrombocytopenia
Immature platelet fraction
Automation
INTRODUCTION
Thrombocytopenia is not a disease entity by itself, but a finding that may result from several disease processes. Platelet counts below 150,000/x00B5;L define thrombocytopenia, but they do not reveal the underlying pathology.[1] The causes of thrombocytopenia can be grouped into three major categories based on the causative process, as due to increased destruction, decreased production, or splenic sequestration/abnormal pooling.[2] The assessment of the thrombopoietic activity in the bone marrow is necessary for correct diagnosis and treatment in thrombocytopenic patients.
For a long time, bone marrow aspiration remained the gold standard method for evaluating the cause of thrombocytopenia. However, this procedure is invasive, time consuming, as well as carries an overt risk of bleeding diathesis in critical thrombocytopenia cases.[3] Other serological tests (for infectious diseases), platelet associated immunoglobulin G (for Immune Thrombocytopenic Purpura (ITP)), and molecular markers for disseminated intravascular coagulation are used in evaluating thrombocytopenic patients but they are relatively costly.[4]
With the availability of automated analyzers, new indices related to platelet count are being estimated. Recently, immature platelet fraction (IPF) has been investigated as a prospective platelet activation marker.[5] It is an automated detection of reticulated platelets in peripheral blood. The IPF is identified by flow cytometric techniques and the use of nucleic acid-specific dye in the reticulocyte/optical platelet channel. The flow cytometric IPF determination uses florescent dyes – polymethine and oxazine, which penetrate the cell membrane staining the RNA in the red blood cells (RBC) and immature/reticulated platelets.[6] Several clinical papers on reticulated platelet analysis have shown that in thrombocytopenia, platelet RNA content correlates directly with megakaryocyte activity. The number of reticulated platelets increases when platelet production rises and decreases when production falls.[7] This can help in determining whether the thrombocytopenia is central or peripheral without the need for bone marrow examination. Our study attempts to find the predictive value of IPF in differentiating hyperdestructive thrombocytopenia with hypoproductive thrombocytopenia.
MATERIALS AND METHODS
The study was a prospective observational study conducted in the Department of Pathology, Atal Bihari Vajpayee Institute of Medical Sciences and Dr. Ram Manohar Lohia Hospital, Delhi, for 9 months. A total of 70 cases were included in the study. An equal number of healthy age and gender matched controls were also enrolled. All patients with hematological disease with platelet counts <1.5 lakhs/x00B5;L and confirmed on peripheral smear examination have been included after careful bone marrow examination. Patients with pseudothrombocytopenia were excluded from the study. Healthy age- and gender-matched individuals with hemoglobin, total leucocyte count, and platelet counts within the normal range were taken as controls. Blood samples from the study population were collected on the same day of the bone marrow procedure in Ethylenediamineacetic acid (EDTA) acid tubes and processed within 4 h. The platelet count and IPF (using SYSMEX 1000N hematology analyzer according to the manufacturer’s instructions) were noted and entered into Excel spreadsheets for 70 subjects with thrombocytopenia and an equal number of healthy age matched controls. The peripheral smears, bone marrow aspirates, and biopsy slides were also evaluated. The clinical diagnosis, diagnosis on bone marrow examination along with IPF% of peripheral blood sample was entered into an excel sheet. According to the bone marrow findings, the cases were grouped into Group 1 with peripheral thrombocytopenia (platelet hyperdestruction), Group 2 with central thrombocytopenia (hypoproductive group), and Group 3 with megaloblastic anemia. The megaloblastic group was separated from the hypoproductive group because the etiology of thrombocytopenia in megaloblastic anemia has been postulated as hypoproduction in some studies and as ineffective erythropoiesis in other studies.[8] Appropriate statistical tests were applied.
Statistics
Sample size calculation was based on the assumptions of minimum 80% power and 5% significance level (significant at 95% confidence level). Assuming a 95% confidence level and a margin of error (confidence interval) of ±10%, the sample size calculated is 61 subjects needed for the study. The cases were then categorized based on the etiopathogenesis of thrombocytopenia into Groups 1, 2, and 3. Categorical variables were presented in number and percentage (%), and continuous variables were presented as mean ± standard deviation. Qualitative variables were compared using the Chi-square test/Fisher’s exact test. Quantitative variables were compared using unpaired t-test/Mann–Whitney test. P < 0.05 was considered significant at a 95% confidence level. Data were analyzed using the Statistical Package for the Social Sciences (SPSS) 24.0 (SPSS Inc., Chicago, IL). The receiver operating characteristic (ROC) curve was obtained.
RESULTS
A total of 70 cases with thrombocytopenia on which bone marrow procedure was performed were included in this study. There were 49 (70%) males and 21 (30%) females in the study group. The patient’s age ranged from 3 years to 75 years. The median age was 29 years. Seventy healthy controls with normal hemoglobin, total leukocyte count, and white blood count were also included in the study. The median age was 32 years. Cases were classified based on etiopathogenesis into three groups-hyperdestructive, hypoproductive, and megaloblastic. There were 34 (48.6%) cases of hyperdestructive etiology (Group 1), 27 (38.5%) cases of hypoproductive etiology (Group 2), and 9 (12.9%) cases of megaloblastic anemia (Group 3). Table 1 shows the etiological distribution of cases among three groups.
| Study groups | Group 1 (Cases with hyperdestructive etiology) | Group 2 (Cases with hypoproduction etiology) | Group 3 megaloblastic anemia |
|---|---|---|---|
| No. of cases | 34 (48.6%) | 27 (38.5%) | 9 (12.9%) |
| Etiology | Infections (20 cases) ITP (9 cases) hemolytic anemia (2 cases) Rheumatoid arthritis (1 case) Rheumatic heart disease (1 case) |
Acute leukemia (20 cases) Plasma cell dyscrasia (4 cases) Aplastic anemia (3 cases) |
ITP: Immune Thrombocytopenic Purpura
The comparison of platelet counts and IPF% among controls and different study groups is shown in Tables 2 and 3, respectively. The range of IPF% in controls was 0.6–6.8%. In the hypoproductive group, the IPF% ranged from 0.2–16.9% to 2.1–37.7% in the hyperdestructive group.
| Total platelet count (/µL) | Group | |||
|---|---|---|---|---|
| Controls | Group 1 (Hyperdestructive etiology) | Group 2 (Hypoproductive etiology) | Group 3 (Megaloblastic anemia) | |
| Mean (SD) | 230,471.43±53,893,54 | 37,956.52±40,891.74 | 48125.0±41828.02 | 59,966,67±40,933.49 |
| Median (IQR) | 221,500 (187250–270,400) | 20,000 (10,000–60,000) | 30,000 (20,000–87,500) | 50,000 (20,000–82,250) |
| Range | 1,50,000–3,99,000 | 2,000–1,20,000 | 10,000–1,30,000 | 10,000–1,40,000 |
SD: Standard deviation, IQR: Interquartile range
| IPF (%) | Group | |||
|---|---|---|---|---|
| Controls | Group 1 (Hyperdestructive etiology) | Group 2 (Hypoproductive etiology) | Group 3 (Megaloblastic anemia) | |
| Mean (SD) | 3.19±1.67 | 13.37±8.18 | 5.0±4.23 | 9.44±12.97 |
| Median (IQR) | 2.8 (1.97–4.65) | 10.6 (8–16.52) | 3.6 (1.92–7.72) | 6.25 (2.82–10.3) |
| Range | 0.6–6.8 | 2.1–37.7 | 0.2–16.9 | 0.1–64.3 |
IPF: Immature platelet fraction, SD: Standard deviation, IQR: Interquartile range
A significant difference was seen in the platelet counts of cases with controls. The IPF% was significantly higher in cases with thrombocytopenia than controls. Furthermore, a statistically significant difference in IPF% was noted for Group 1 with Group 2 and Group 3, with P < 0.001 and <0.01, respectively. IPF% was significantly higher in cases with increased platelet destruction than with decreased platelet production [Table 4]. The optimal IPF value for discriminating between Groups 1 and 2 was derived using the ROC curve [Figure 1]. An IPF value of 8.20% was calculated as the cutoff value for differentiating hyperdestructive thrombocytopenia from hypoproductive thrombocytopenia with a sensitivity of 75% and specificity of 87.5%.
| P-value | ||||||
|---|---|---|---|---|---|---|
| Controls and Group 1 | Controls and Group 2 | Controls and Group 3 | Group 1 and Group 2 | Group 1 and Group 3 | Group 2 and Group 3 | |
| Platelet count(/µL) | <0.001 | <0.001 | <0.001 | 0.17 | 0.01 | 0.25 |
| IPF (%) | <0.001 | 0.16 | <0.001 | <0.001 | <0.01 | 0.18 |
Group 1: Cases with hyperdestructive etiology, Group 2: Cases with hypoproductive etiology, Group 3: Cases of Megaloblastic anemia. IPF: Immature platelet fraction
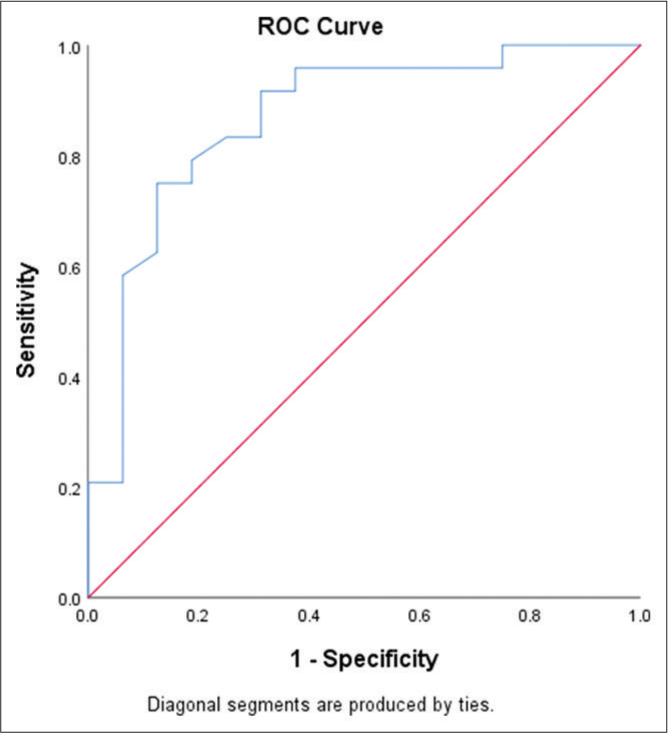
- Receiver operating characteristic curve (ROC) using immature platelet fraction for prediction of hyperdestructive etiology.
DISCUSSION
The etiological diagnosis of thrombocytopenia (Total Platelet count <150,000/x00B5;L) requires laboratory confirmation along with clinical assessment. Till now, bone marrow examination is considered as a gold standard for confirmation of the diagnosis, which is an invasive and time consuming procedure. Our study was aimed at analyzing and establishing the usefulness of IPF as a newer, rapid, and inexpensive automated platelet parameter for the diagnosis of thrombocytopenia. IPF is expressed in percentage, which is a measure of reticulated platelets which are newly released platelets with increased RNA content as compared to mature platelets. They can be considered platelet analogs of RBC reticulocytes and reflect underlying thrombopoietic activity.[9] In our study, the normal range of IPF was calculated from 70 age matched healthy individuals and was found to be 0.6–6.8% with a mean value of 3.19 ± 1.67 and median of 2.8 (Interquartile range [IQR] = 1.97– 4.65). This is in concordance with studies done by Goel et al.,[10] Briggs et al.,[6] and Dadu et al.[11] which calculated their normal reference IPF% as 0.7–5.7%, 1.1–6.7%, and 0.7–4.3%, respectively. IPF was significantly higher in cases of the hyperdestructive group (mean IPF = 13.37 ± 8.18) as compared to the hypoproductive group (mean IPF = 7.90 ± 10.90) and controls (mean IPF 3.19 ± 1.67) with P-value being <0.001 between the two groups. ROC curve analysis revealed an optimal cutoff value of 8.20% for differentiating between hyperdestructive and hypoproductive thrombocytopenia with a sensitivity of 75% and specificity of 87.5%. This is consistent with studies done by Goel et al.[10] and Jung et al.[12] Goel et al.[10] also classified thrombocytopenic patients into hypoproductive and hyperdestructive groups and found significantly higher mean IPF in the hyperdestructive group (13.4%) as compared to the hypoproductive group (4.6%). The optimal cutoff between both groups is 5.95%, with a sensitivity of 88% and specificity of 75.9%. Studies done by Cho et al.[13] had also evaluated the discriminatory power of IPF in discriminating hyperdestructive and hypoproductive causes of thrombocytopenia and found that IPF was significantly higher in the hyperdestructive group (Mean IPF: 6.2% [IQR 4.3–10.3%] than both control groups (Mean IPF:1.8% [1.32.4%]) and hypoproductive group (mean 1.8% [0.9–2.3%]) with all P < 0.001. However, similar to our study, they did not find a significant difference between the hypoproductive group and the control group (P = 0.18). Ashraf et al.[14] had found higher IPF (mean: 25.5% and IQR = 15.2–39.3%) in the hyperdestructive group (peripheral thrombocytopenia) as compared to the hypoproductive group (mean: 8.2% and IQR = 4.6–16.7%) with a statistically significant difference (P < 0.001). In our study, we evaluated the megaloblastic group separate from the hypoproductive and hyperdestructive group, similar to the studies done by Akula et al.[7] and Rajashekar et al.[8] In our study, the megaloblastic group constituted 41% of cases [Table 1]. The median IPF was 6.25%, with a range of 0.1–64.3% [Table 3]. The IPF in the megaloblastic group was significantly higher as compared to other hypoproductive causes (P < 0.01). This suggested a mechanism other than hypoproduction for thrombocytopenia in megaloblastic anemia. Akula et al.[7] had also attempted to study the role of IPF in the diagnosis and prognosis of thrombocytopenic groups. This study also evaluated IPF in megaloblastic groups in addition to hypoproductive and hyperdestructive groups and found the IPF range of hyperdestructive group 4.4– 55.6%, hypoproductive 2.8–7.4%, and megaloblastic group as 5.3–30.7%. Hence, the megaloblastic group needs to be separated from the hypoproductive and hyperdestructive group as IPF% was significantly higher, and further studies are needed for assessment in megaloblastic patients.
CONCLUSIONS
IPF is a simple, inexpensive, rapid, and non-invasive automated marker for the etiology of thrombocytopenia. By differentiating between hypoproductive and hyperdestructive causes of thrombocytopenia, it has a definite role in the initial assessment of the etiology of thrombocytopenia and, hence, can be integrated as a standard parameter to evaluate the thrombopoetic state of the bone marrow.
The study is, however, limited by a smaller sample size, and a larger sample size needs to be studied for a definitive conclusion.
Acknowledgment
We acknowledge the support of our colleagues and technical staff of the Department of Pathology for their guidance, as well Department of Internal Medicine and Pediatrics.
Author contributions
HB: Study conception and design, data collection and selection of cases, analysis and interpretation of results, draft manuscript preparation; VK, TR, MS: Study conception and design, analysis and interpretation of results, review of manuscript; PS, AH: Data collection and selection of cases, clinical correlation.
Ethical approval
The research/study approved by the Institutional Review Board at Atal Bihari Vajpayee Institute of Medical Sciences and Dr Ram Manohar Lohia Hospital New Delhi, number 575 (18/2022)/IEC/ABVIMS/RMLH/1035, dated 27th August 2022.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Immature platelet count: A simple parameter for distinguishing thrombocytopenia in pediatric acute lymphocytic leukemia from immune thrombocytopenia. Pediatr Blood Cancer. 2011;57:641-7.
- [CrossRef] [PubMed] [Google Scholar]
- Role of platelet parameters in thrombocytopenia. Int J Clin Diagn Pathol. 2019;2:103-6.
- [CrossRef] [Google Scholar]
- Role of platelet indices in differentiating hypoproductive and hyperdestructive thrombocytopenia. Ann Pathol Lab Med. 2017;4:A288-91.
- [CrossRef] [Google Scholar]
- Platelet size deviation width, platelet large cell ratio, and mean platelet volume have sufficient sensitivity and specificity in the diagnosis of immune thrombocytopenia. Br J Haematol. 2005;128:698-702.
- [CrossRef] [PubMed] [Google Scholar]
- Role of platelet parameters in dengue positive cases-an inter observational study. Int J Health Sci Res. 2016;6:74-7.
- [Google Scholar]
- Assessment of an immature platelet fraction (IPF) in peripheral thrombocytopenia. Br J Haematol. 2004;126:93-9.
- [CrossRef] [PubMed] [Google Scholar]
- Role of immature platelet fraction in diagnosis and prognosis of thrombocytopenic groups. Indian J Pathol Res Pract. 2020;9:49-53.
- [CrossRef] [Google Scholar]
- Evaluation of thrombocytopenia in megaloblastic anemia by platelet indices and megakaryocytes-comparison with hypoproduction and hyperdestruction. Natl J Lab Med. 2017;6:PO18-22.
- [Google Scholar]
- Automatic detection of immature platelets for decision making regarding platelet transfusion indications for pediatric patients. Transfus Apher Sci. 2008;38:127-32.
- [CrossRef] [PubMed] [Google Scholar]
- Immature platelet fraction : Its clinical utility in thrombocytopenia patients. J Lab Physicians. 2021;13:214-8.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of the immature platelet fraction as an indicator of platelet recovery in dengue patients. Int J Lab Hematol. 2014;36:499-504.
- [CrossRef] [PubMed] [Google Scholar]
- Immature platelet fraction: Establishment of a reference interval and diagnostic measure for thrombocytopenia. Korean J Lab Med. 2010;30:451-9.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical usefulness of the simple technique to diagnose thrombocytopenia using immature platelet fraction. Korean J Lab Med. 2007;27:1-6.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of immature platelet fraction (IPF) in patients with central thrombocytopenia and peripheral thrombocytopenia. J Coll Physicians Surg Pak. 2020;30:796-800.
- [CrossRef] [PubMed] [Google Scholar]






