Translate this page into:
Role of p16/INK4a and Ki-67 as specific biomarkers for cervical intraepithelial neoplasia: An institutional study
Address for correspondence: Dr. Ankitha Hebbar, Senior Resident, Department of Pathology, No. 321, 5th main, 6th cross, Arekere Mico Layout, Bannerghatta Road, Bangalore - 560 076, Karnataka, India. E-mail: ankitha.hebbar@gmail.com
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
BACKGROUND:
P16/INK4a and Ki-67 have emerged as important biomarkers for the detection of high-risk human papilloma virus (HR-HPV) associated dysplastic changes in the cervical biopsy samples. The increasing inter- and intra-observer variability in the diagnosis of dysplastic lesions and immature squamous metaplasia on histopathology has led to the advent of these biomarkers. This study was taken up with an aim to study their role in increasing the diagnostic accuracy in equivocal cases on histopathology.
MATERIALS AND METHODS:
Fifty cervical biopsy specimens were stained with p16/INK4a and Ki-67 consisting of 10 cases each of cervical intraepithelial neoplasia (CIN I/II/III) along with five cases of squamous metaplasia. Histopathological diagnosis was considered as the gold standard. Statistical analysis was done by kappa statistics, and P value was calculated.
RESULTS:
The sensitivity and specificity of p16/INK4a and Ki-67 were 76.2%, 87.5%, 90.5%, and 87.5%, respectively. The overall agreement of both the immunostains with histopathological diagnosis was statistically significant (P < 0.05) and the diagnostic accuracy improved when both the stains were used in conjunction.
CONCLUSION:
Ki-67 and p16/INK4a can be used as complimentary tests in differentiating dysplastic and nondysplastic lesions and help in confirming the histopathological diagnosis. They aid in recognition of dysplasias caused by HR-HPV, which have higher tendency to progress to neoplasia. However, further research is advocated before the widespread use of these markers for screening of dysplasias.
Keywords
Cervical intraepithelial neoplasia
human papilloma virus
Ki-67
p16/INK4a
Introduction
Cervical carcinoma is the third most common malignancy in women worldwide accounting for 7.9% of all malignancies and the second most common malignancy in women in India. It is associated with agonizing morbidity and high mortality.[1] Another cause for concern is the increasing prevalence of HIV in the Indian subcontinent as the progression of human papilloma virus (HPV) infection to neoplasia is found to be more rapid in HIV-infected women.[2] Cervical carcinoma is, however, a preventable disease. Early detection and treatment are the key to reduction in morbidity and mortality due to cancer.[3] Cervical carcinoma screening has been performed for more than 50 years by the conventional Pap smears, which has substantially reduced the incidence of cervical neoplasia, especially in developed countries by successfully identifying the asymptomatic women with preneoplastic lesions and early cervical neoplasia.[4]
Although the screening programs using Pap smears are highly successful, a substantial number of cases of cervical cancer have still been missed which can be attributed to false negative test results due to sampling errors, inter- and intra-observer variability. Pap smears are reported to have low sensitivity of 66% for high-grade squamous intraepithelial lesions (HSILs). In addition, a large number of false positive test results have been reported due to the presence of reserve cell hyperplasia, atypical immature metaplasia, or inflammatory atypia on smears. These have led to unnecessary overtreatment with repeat Pap smears or even surgical intervention. The use of hybrid capture 2 for assessment of high-risk-HPV (HR-HPV) has the advantage of having high sensitivity and aids in triaging of ASCUS smears but has low specificity for cervical neoplasia. Histopathological diagnosis of cervical biopsy samples, which is the current gold standard, is also observed to have significant interobserver discrepancies. Therefore, there is a need for additional sensitive and specific biomarkers to improve cervical cancer screening which can improve standardization and quality control of histopathological diagnosis.[56]
The most common cause of cervical carcinoma and dysplasia is HPV, which is categorized as low risk and HR types based on their association with cervical carcinoma. The HR-HPV types 16 and 18 are causative of 99% of the cervical carcinomas. The HPV oncoproteins most commonly associated with the pathogenesis of cervical neoplasia are E6 and E7. These oncoproteins bind to regulatory tumor suppressor proteins p53 and Rb and cause their degradation and functional inactivation, respectively. Binding of the E7 oncoprotein to Rb releases the E2F from complex with hypophosphorylated Rb and causes progression of cell cycle. The functional inactivation of Rb thus causes overexpression of the CDK inhibitor p16/INK4a through negative feedback control to check the cell proliferation through regulation of CDK4 and 6. Therefore, HPV-mediated cervical neoplasia and dysplasia overexpress p16/INK4a. In addition, Ki-67 which is a reliable marker of DNA replication, and cell proliferation is often expressed in cervical neoplasia and dysplasia.[567]
In this study, we performed immunohistochemical staining of formalin-fixed paraffin embedded samples of cervical biopsy with an aim to analyze the expression of p16/INK4a and Ki-67 immunostains on nonneoplastic, dysplastic and neoplastic specimens and to study their role in increasing the diagnostic accuracy in equivocal cases on histopathology. The degree of staining by p16/INK4a was compared with that of Ki-67 immunostain in different lesions. The role of HPV in the etiology of dysplasia and carcinoma was evaluated by assessing the degree of immunoreactivity for p16/INK4a.
Materials and Methods
Case selection
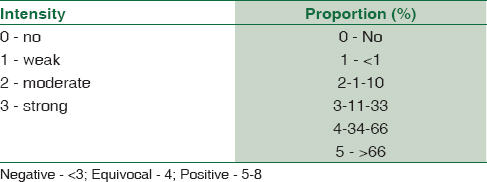
Cervical biopsy specimens (n = 50) from women aged between 20 and 60 years were obtained from the Department of pathology, ESIC MC and PGIMSR, Bengaluru, which were randomly selected for the study from the archives during 2014 and 2015. The biopsy specimens were stained with H and E stain. The slides were studied in detail for the presence or absence of dysplasia and reported according to the cervical intraepithelial neoplasia (CIN) system of reporting proposed by Richart[8] [Table 1].

The histopathological diagnosis comprised 3 cases of negative for dysplasia, 5 cases of squamous metaplasia with/without atypia, 10 cases each of CIN I, CIN II, and CIN III, [Figures 1a, 2a, 3a] 6 cases each of squamous cell carcinoma and adenocarcinoma [Figures 4a and 5a] - a total of 50 cases. H and E diagnosis was considered as the gold standard. All the specimens were immunostained for Ki-67 and p16/INK4a.
![(a) Chronic cervicitis - Section shows normal squamous epithelial lining with sparse inflammatory infiltrate in the stroma (HPE [H and E, ×10, ×40]). (b) Negative immunostaining in a case of chronic cervicitis (p16INK4a [IHC, ×10, ×40). (c) Negative immunostaining in a case of chronic cervicitis (Ki-67 [IHC, ×10, ×40])](/content/164/2017/9/2/img/JLP-9-104-g002.png)
- (a) Chronic cervicitis - Section shows normal squamous epithelial lining with sparse inflammatory infiltrate in the stroma (HPE [H and E, ×10, ×40]). (b) Negative immunostaining in a case of chronic cervicitis (p16INK4a [IHC, ×10, ×40). (c) Negative immunostaining in a case of chronic cervicitis (Ki-67 [IHC, ×10, ×40])
![(a) Cervical intraepithelial neoplasia I - Section shows crowding of cells in the basal third of the epithelium. Nuclei are enlarged, hyperchromatic, and show loss of polarity. Few cells exhibiting koilocytotic atypia are seen (HPE [H and E, ×10, ×40]). (b) Section shows moderate nuclear and cytoplasmic staining of the lower 1/3 of epithelium in cervical intraepithelial neoplasia I; Score 5 (p16INK4a [IHC, ×10, ×40]). (c) Positive immunostaining in the nuclei of lower two-third of the epithelium in cervical intraepithelial neoplasia I; 60% positivity, Score 3 (Ki-67 [IHC, ×10, ×40])](/content/164/2017/9/2/img/JLP-9-104-g003.png)
- (a) Cervical intraepithelial neoplasia I - Section shows crowding of cells in the basal third of the epithelium. Nuclei are enlarged, hyperchromatic, and show loss of polarity. Few cells exhibiting koilocytotic atypia are seen (HPE [H and E, ×10, ×40]). (b) Section shows moderate nuclear and cytoplasmic staining of the lower 1/3 of epithelium in cervical intraepithelial neoplasia I; Score 5 (p16INK4a [IHC, ×10, ×40]). (c) Positive immunostaining in the nuclei of lower two-third of the epithelium in cervical intraepithelial neoplasia I; 60% positivity, Score 3 (Ki-67 [IHC, ×10, ×40])
![(a) Cervical intraepithelial neoplasia III - Section shows crowding of cells in the full thickness of the epithelium. Nuclei are enlarged, hyperchromatic, and show loss of polarity. Few mitotic figures noted (HPE [H and E, ×10, ×10]). (b) Section shows diffuse strong positive cytoplasmic and nuclear staining in full thickness of the epithelium in cervical intraepithelial neoplasia III; Score 8 (p16INK4a [IHC, ×10, ×10]). (c) Diffuse strong nuclear positive immunostaining in full thickness of the epithelium in cervical intraepithelial neoplasia III; 80% positivity, Score 3 (Ki-67 [IHC, ×10, ×40])](/content/164/2017/9/2/img/JLP-9-104-g004.png)
- (a) Cervical intraepithelial neoplasia III - Section shows crowding of cells in the full thickness of the epithelium. Nuclei are enlarged, hyperchromatic, and show loss of polarity. Few mitotic figures noted (HPE [H and E, ×10, ×10]). (b) Section shows diffuse strong positive cytoplasmic and nuclear staining in full thickness of the epithelium in cervical intraepithelial neoplasia III; Score 8 (p16INK4a [IHC, ×10, ×10]). (c) Diffuse strong nuclear positive immunostaining in full thickness of the epithelium in cervical intraepithelial neoplasia III; 80% positivity, Score 3 (Ki-67 [IHC, ×10, ×40])
![(a) Squamous cell carcinoma - Section shows a tumor displaying features of invasive squamous cell carcinoma (HPE [H and E, ×10, ×10]). (b) the tumor shows strong diffuse cytoplasmic and nuclear positivity in SCC; Score 8 (p16INK4a [IHC, ×10, ×10]). (c) Diffuse strong nuclear positivity in the tumor cells in SCC; 90% positivity, Score 3 (Ki-67 [IHC, ×10, ×10])](/content/164/2017/9/2/img/JLP-9-104-g005.png)
- (a) Squamous cell carcinoma - Section shows a tumor displaying features of invasive squamous cell carcinoma (HPE [H and E, ×10, ×10]). (b) the tumor shows strong diffuse cytoplasmic and nuclear positivity in SCC; Score 8 (p16INK4a [IHC, ×10, ×10]). (c) Diffuse strong nuclear positivity in the tumor cells in SCC; 90% positivity, Score 3 (Ki-67 [IHC, ×10, ×10])
![Adenocarcinoma - Section shows a tumor displaying features of invasive endocervical adenocarcinoma (HPE [H and E, ×10, ×10]). (b) Tumor cells show strong diffuse cytoplasmic and nuclear positivity in adenocarcinoma; Score 8 (p16INK4a [IHC, ×10, ×10]). (c) Diffuse strong nuclear positive immunostaining in adenocarcinoma; 90% positivity, Score 3 (Ki-67 [IHC, ×10, ×10])](/content/164/2017/9/2/img/JLP-9-104-g006.png)
- Adenocarcinoma - Section shows a tumor displaying features of invasive endocervical adenocarcinoma (HPE [H and E, ×10, ×10]). (b) Tumor cells show strong diffuse cytoplasmic and nuclear positivity in adenocarcinoma; Score 8 (p16INK4a [IHC, ×10, ×10]). (c) Diffuse strong nuclear positive immunostaining in adenocarcinoma; 90% positivity, Score 3 (Ki-67 [IHC, ×10, ×10])
Immunohistochemical staining
Immunohistochemical staining of the formalin-fixed paraffin embedded biopsy specimens was performed with p16/INK4a and Ki-67 antibodies according to the instructions by the manufacturer on 3 μm thick sections on silane coated slides. Antigen retrieval was carried out with ethylene diamine tetra-acetic acid buffer using pressure cooker after deparaffinizing the sections and rehydration through graded alcohols. Horseradish peroxidase method was used. Approximately, 100 microliters of the primary antibody [Table 2] was applied to each slide following power block. Enhancer was applied followed by secondary antibody. Diaminobenzidine was the chromogen used with H2O2 as the substrate. The slides were counterstained in hematoxylin, dehydrated through graded alcohols, cleared in xylene, and coverslipped with DPX. Cases of chronic cervicitis were taken as negative control, and cases of SCC and adenocarcinoma were taken as positive control.

The results for p16/INK4a and Ki-67 were scored by a semi-quantitative scoring system[910] as mentioned in Tables 3 and 4. Histological diagnosis was considered as the gold standard, and sensitivity and specificity were calculated. Statistical analysis was performed with Kappa statistics to assess associations between the two tests (<0.20 - Poor, 0.21–0.40 - Fair, 0.41–0.60 - Moderate, 0.61–0.80 - Good, and 0.81–1.00 - very good) and a P < 0.05 was considered as statistically significant.

Results
The age of patients in our study ranged from 31 to 70 years. The majority of the patients were in the age group of 41–50 years with a mean age of 47 years [Table 5].

p16 immunostaining
The p16/INK4a immunoexpression was evaluated in all cases and correlated with the degree of cervical neoplasia. All the inflammatory samples showed no expression of p16/INK4a [Figure 1b] and one case reported as atypical squamous metaplasia showed positive expression (1/5, 20%). Out of the 10 CIN I lesions, five showed positivity with ≥5 expression in the lower third of the epithelium, whereas the upper two-thirds showed absent staining [Figure 2b]. The staining pattern was predominantly cytoplasmic in most of the samples. In cases of high-grade dysplasia, of the 10 CIN II cases, 7 (70%) showed strong positivity in the lower two-thirds of the epithelium and one case showed focal weak staining, considered equivocal. CIN III lesions were mostly, strongly positive in the entire thickness of the epithelium in nine cases (90%) while one case was with equivocal staining [Figure 3b]. All the invasive squamous cell carcinomas showed diffuse strong positive staining (100%) [Figure 4b]. Adenocarcinoma cases also showed diffuse strong positivity [Figure 5b] in all cases (83%) except one which was a case of well-differentiated adenocarcinoma [Table 6].
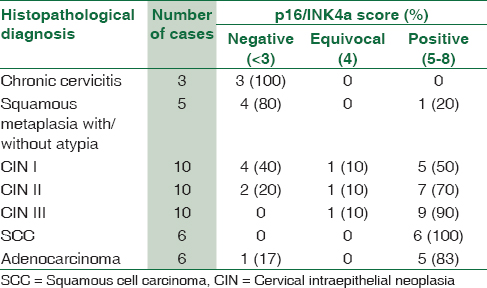
The sensitivity and specificity of p16 staining were 76.2% and 87.5% with 96.9% positive predictive value (PPV) and 41% negative predictive value (NPV), respectively. The overall agreement between p16 and H and E diagnosis was 78% (κ = 0.438, P = 0.000491).
Ki-67 immunostaining
Ki-67 immunostaining was negative in chronic cervicitis cases [Figure 1c] showed positive expression in 70% of CIN I cases in the basal half of the epithelium [Figure 2c] in contrast to 40% of cases of squamous metaplasia with atypia. The Ki-67 expression in these cases can be attributed to hyperplasia secondary to inflammation. In cases of CIN II and III [Figure 3c], there was more intense positive staining seen in the full thickness of the epithelium. Invasive carcinomas [Figures 4c and 5c] showed diffuse and strong expression of Ki-67 in all cases [Tables 7 and 8]. The sensitivity and specificity of Ki-67 staining were 90.5% and 87.5%, respectively with 95% PPV and 60% NPV. The overall agreement of Ki-67 staining with H and E diagnosis was 88% (κ = 0.595, P = 0.000022).
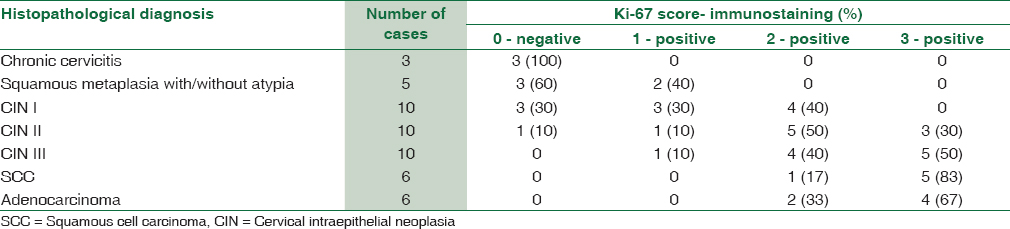

All the p16 positive cases were also positive for Ki-6 and none was negative for Ki-67. The agreement between p16 and Ki-67 immunostaining was 86% (κ = 0.653, P = 0.000001) [Table 9].

Discussion
The increasing search for better biomarkers to screen for cervical neoplasia due to marked inter-and intra-observer variability in the interpretation of Pap smears and cervical biopsy specimens has led to the study of p16/INK4a and Ki-67 as valuable biomarkers to improve the sensitivity and specificity of these tests and aid in the diagnosis of equivocal cervical biopsies.[711] The low sensitivity of Pap smears and low specificity of HPV testing by HybridCapture2 are other factors which signifies the importance of these biomarkers. The role of HR-HPV in the causation of cervical dysplasias and neoplasia further helps to identify the utility of these markers, especially p16/INK4a as its high expression is known to correlate with the prevalence of HR-HPV types [Figure 6].[612]
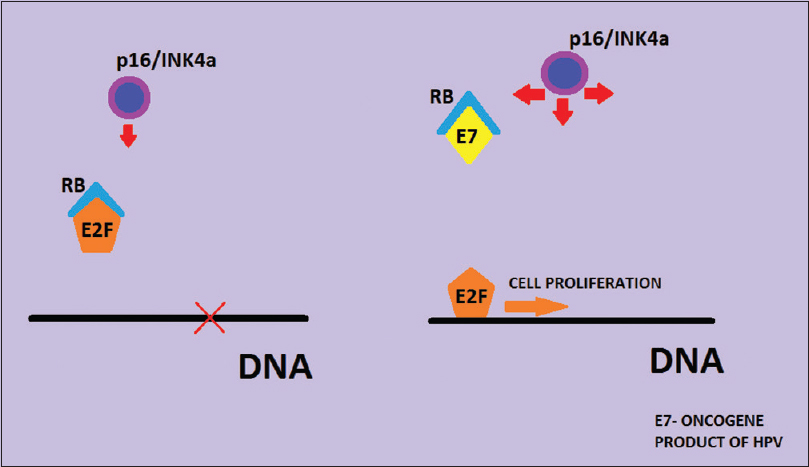
- Mechanism of p16/INK4a overexpression in cervical human papillomavirus infection
The grade of dysplasia had been found to correlate well with the Ki-67 expression which is why it has been increasingly studied for the evaluation of low-grade lesions of the cervix. The high specificity and reproducibility with this staining have led to its increasing utility in screening. Diffuse positive staining is found to be consistent with CIN, but Ki-67 staining cannot differentiate between dysplasia and immature squamous metaplasia. However, Ki-67 staining is advantageous over HPV testing as subclinical HPV infections show negative staining, and it is a simple, low-cost laboratory technique.[711]
In our study, CIN I showed the least immunoexpression for p16/INK4a and Ki-67 antibodies. P16/INK4a was a useful diagnostic adjunct for diagnosing CIN and discriminating it from nonneoplastic lesions. CIN II and III cases showed more diffuse and consistent staining with both Ki-67 and p16/INK4a. A study by Benevolo et al. suggested that CIN I lesions which were positive for p16/INK4a had a higher tendency to progress to CIN II/III indicating the importance of p16 in the evaluation of CIN I.[13] Few cases of immature squamous metaplasia showed positive staining with Ki-67, which could be attributed to regeneration or hyperplasia. Thus, in such cases, Ki-67 alone cannot differentiate between dysplasia and immature squamous metaplasia. The sensitivity and specificity with Ki-67 staining were 90.5% and 87.5%, respectively.
Immunostaining for p16/INK4a had lesser variability and showed negative staining in cases of immature squamous metaplasia except one case which was also positive for Ki-67, which is compatible with CIN II. In our study, sensitivity and specificity with p16/INK4a immunostaining were 76.2% and 87.5%, respectively. Many studies have signified the importance of p16 as a sensitive and specific biomarker with better predictive value than HPV-DNA testing for HSIL.[11] The results of our study are concordant with the results of other studies as mentioned in Table 10. Both the immunostains had moderate to good agreement with the histopathological diagnosis and the accuracy was better when both the stains were used together.
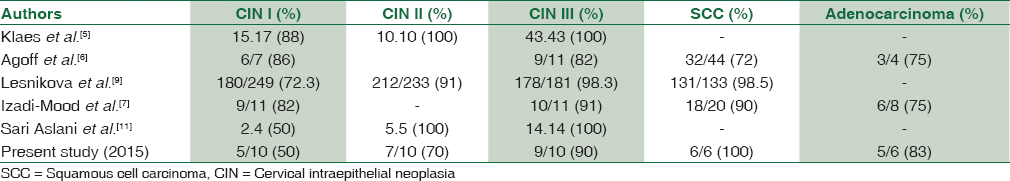
A study by Agoff et al. in 2003 included 597 specimens of cervical biopsy/LEEP and compared the p16/INK4a staining with Ki-67 and analyzed the expression of p16/INK4a in relation to the degree of neoplasia. They also correlated the p16/INK4a staining with HR-HPV types by in situ hybridization and HybridCapture2/polymerase chain reaction (PCR) techniques. They observed that the expression of p16/INK4a increased with an increase in the grade of dysplasia and neoplasia. They found positive staining for p16/INK4a in 57%, 75% and 91% cases of CIN I, CIN II and CIN III respectively which is close to the findings in the current study. p16/INK4a was observed to be more specific for neoplasia and dysplasia than Ki-67 and Hybrid Capture2/PCR for HR-HPV types in their study.[6]
The lower expression of p16/INK4a in low-grade lesions in our study could be due to the causation of these lesions by low-risk HPV types whose E7 protein has lower affinity for Rb than that of HR-HPV, and hence, the absence of overexpression of p16/INK4a as stated by Agoff et al.[6] Also, we found few cases of squamous metaplasia with atypia showing positivity with Ki-67 antibodies which could be due to the higher proliferation rate in these cases secondary to inflammation/reactive changes.
Klaes et al., in their study, found a very strong expression in dysplastic and neoplastic epithelium which was clearly distinguished from the adjacent normal epithelium. They observed diffuse strong immunostaining with p16/INK4a in 60% and 100% of CIN I and CIN II/III lesions respectively which correlates well with our study results. They found a good correlation of CIN I lesions which were HR-HPV positive with intense diffuse p16/INK4a immunostaining while the LR-HPV types were consistently negative for p16.[5]
Few of the authors like Ikenberg et al. and Roelens et al. have studied the dual-staining of Pap cytology smears with p16/Ki-67 and found superior sensitivity over Pap cytology in detecting dysplasia suggesting a role in screening and triaging the ASCUS and LSIL cases on cervical cytology.[1415] Liu et al. have studied the p16INK4a expression on cell block sections obtained from liquid-based cytology specimens and suggested p16 as a sensitive marker for the confirmation of the diagnosis of ASC-H.[16] Duncan et al. have similarly evaluated the use of p16INK4a in the triage of Pap smears demonstrating ASCUS and suggested that p16 had better correlation with tissue follow-up than Hybrid Capture 2.[12]
Atypical immature metaplasia falls short of the diagnosis CIN I but has resemblance to CIN III and has significant inter- and intra-observer variability. The use of biomarkers like Ki-67, p16/INK4a along with CK17 can distinguish these lesions to increase the diagnostic accuracy in equivocal cases. A study by Sari Aslani et al. on 77 cervical biopsy/curettage specimens using Ki-67, p16/INK4a, and CK17 immunostains found positive staining for Ki-67 in 26.6% of immature squamous metaplasia cases while all those cases were negative for p16/INK4a. They found positive staining for CK17 in all cases of immature squamous metaplasia as it is a marker for reserve cells which gives rise to metaplasia.[11] Some other studies have suggested the reclassification of such immature metaplastic lesions based on negative or positive immunostaining as benign or HSIL, respectively.[17] While few others have laid different protocol for follow-up of cases with atypical squamous metaplasia based on p16 and Ki-67 immunoreactivity and in situ hybridization for HPV.[1819]
The merits of the study are that negative, metaplastic, and dysplastic samples were considered along with invasive carcinoma to study the degree of expression in various lesions for both the markers. The limitations of this study are the limited number of cases studied and that HPV testing was not done in these cases to correlate with the p16/INK4a immunostaining.
Conclusion
Ki-67 and p16/INK4a can be used as complimentary tests for differentiating dysplastic and nondysplastic lesions. They also help in confirming the diagnosis in these cases as different lesions have specific treatment protocols based on the degree of dysplasia. The importance of p16/INK4a in cervix is that it is specific for HR-HPV associated dysplasia and is seen in high-grade lesions and few low-grade lesions with high tendency to progress to a higher grade. However, further research is necessary for the use of these markers with cytological techniques such as liquid-based cytology for the early screening of dysplasia and neoplasia.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 2013. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11. Lyon, France: International Agency for Research on Cancer; Available from: http://www.globocan.iarc.fr
- Virus and cervical cancer: Role and implication: A review. Biomed Res Ther. 2015;2:220-30.
- [Google Scholar]
- Study of a manual method of liquid-based cervical cytology. Indian J Pathol Microbiol. 2008;51:190-4.
- [Google Scholar]
- Preliminary study of a new, fully automated system for liquid-based cytology: The NovaPrep® processor system. Acta Cytol. 2011;55:281-6.
- [Google Scholar]
- Overexpression of p16(INK4A) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer. 2001;92:276-84.
- [Google Scholar]
- p16(INK4a) expression correlates with degree of cervical neoplasia: A comparison with Ki-67 expression and detection of high-risk HPV types. Mod Pathol. 2003;16:665-73.
- [Google Scholar]
- Potential diagnostic value of P16 expression in premalignant and malignant cervical lesions. J Res Med Sci. 2012;17:428-33.
- [Google Scholar]
- Cytology: Diagnostic Principles and Clinical Correlates (3rd ed). China: Saunders Elsevier; 2009.
- p16 as a diagnostic marker of cervical neoplasia: A tissue microarray study of 796 archival specimens. Diagn Pathol. 2009;4:22.
- [Google Scholar]
- Tumor biomarkers in cervical cancer: Focus on Ki-67 proliferation factor and E-cadherin expression. Rom J Morphol Embryol. 2009;50:413-8.
- [Google Scholar]
- Evaluation of Ki67, p16 and CK17 markers in differentiating cervical intraepithelial neoplasia and benign lesions. Iran J Med Sci. 2013;38:15-21.
- [Google Scholar]
- Evaluation of p16INK4a as a diagnostic tool in the triage of Pap smears demonstrating atypical squamous cells of undetermined significance. Cancer. 2008;114:34-48.
- [Google Scholar]
- Immunohistochemical expression of p16(INK4a) is predictive of HR-HPV infection in cervical low-grade lesions. Mod Pathol. 2006;19:384-91.
- [Google Scholar]
- Screening for cervical cancer precursors with p16/Ki-67 dual-stained cytology: Results of the PALMS study. J Natl Cancer Inst. 2013;105:1550-7.
- [Google Scholar]
- p16INK4a immunocytochemistry versus human papillomavirus testing for triage of women with minor cytologic abnormalities: A systematic review and meta-analysis. Cancer Cytopathol. 2012;120:294-307.
- [Google Scholar]
- Immunohistochemical detection of p16INK4a in liquid-based cytology specimens on cell block sections. Cancer. 2007;111:74-82.
- [Google Scholar]
- p16(INK4a) immunostaining as an alternative to histology review for reliable grading of cervical intraepithelial lesions. J Clin Pathol. 2010;63:972-7.
- [Google Scholar]
- P16/Ki-67 immunostaining is useful in stratification of atypical metaplastic epithelium of the cervix. Clin Med Pathol. 2008;1:35-42.
- [Google Scholar]
- p16 and Ki-67 immunostaining in atypical immature squamous metaplasia of the uterine cervix: Correlation with human papillomavirus detection. Arch Pathol Lab Med. 2007;131:1343-9.
- [Google Scholar]





