Translate this page into:
Screening of methicillin-resistant Staphylococcus aureus in healthcare workers and students and its susceptibility to mupirocin in a tertiary care teaching hospital in South India
Address for correspondence: Dr. Jutang Babat Ain Tiewsoh, C/o Mrs. M. Hooroo, Upland Main Road, Laitumkhrah, Shillong - 793 003, Meghalaya, India. E-mail: jutangbabat@gmail.com
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
BACKGROUND:
Staphylococcus is the most common pathogen causing infection in hospitals. They also colonize the healthcare workers who serve as reservoir of infection. Emergence of methicillin-resistant Staphylococcus aureus (MRSA) is a burning issue throughout the world contributing to significant morbidity and mortality. Use of mupirocin to eradicate the carrier state is the need of the hour.
OBJECTIVES:
To screen healthcare workers (HCWs) and medical students for MRSA and to know the susceptibility of mupirocin in this group.
MATERIALS AND METHODS:
A total of 432 students, nursing staff, doctors and house-keeping staff were screened for MRSA for 4 months. The MRSA and methicillin-resistant coagulase-negative Staphylococcus (MRCoNS) isolates were then tested for mupirocin resistance.
RESULTS:
Out of 432 samples, 24 (5.55%) were MRSA and 104 (24.07%) were MRCoNS. Only 4.16% (n = 1) showed high-level resistance to mupirocin among the MRSA isolates, while resistance among MRCoNS was higher at 6.7% (n = 7) for low-level resistance and 17.30% (n = 18) for high-level resistance.
CONCLUSION:
MRSA colonization of HCWs may serve as a source of infection and mupirocin resistance should be screened for all whether working in Intensive Care Units or not and if detected, alternative treatment should be used which will result in appropriate use of this antibiotic for decolonization.
Keywords
Healthcare workers
methicillin-resistant coagulase-negative Staphylococcus
methicillin-resistant Staphylococcus aureus
mupirocin
Staphylococcus aureus
Introduction
Staphylococcus aureus is the most important cause of wound and skin infections. Originally, penicillin was the drug of choice for treatment of serious S. aureus infections, but resistance due to the acquisition of plasmid-borne genetic elements coding for β-lactamase production occurred. Then, semisynthetic penicillinase-resistant penicillins such as oxacillin and methicillin became the drug of choice. Resistance to these also noticed due to the presence of an altered penicillin-binding protein called PBP 2a or PBPs that results from acquisition of chromosomal gene called mec A. The S. aureus strains expressing the mec A determinant are termed methicillin-resistant S. aureus (MRSA). The mec A determinant of S. aureus is also found in methicillin-resistant coagulase-negative Staphylococcus (MRCoNS).[1]
MRSA can colonize the nose and other skin sites without causing infection. MRSA can spread by airborne route but is most commonly spread by the colonized hands of healthcare workers (HCWs). Colonized health workers and students in teaching hospitals may subsequently develop clinical infections and act as reservoirs for infection among vulnerable individuals.[2] Standard MRSA decolonization therapy includes the use of topical application of mupirocin 2%, bacitracin, tea tree oil of Melaleuca alternifolia plant, retapamulin of the pleuromutilins group of antibiotics, chlorhexidine gluconate and sodium hypochlorite. Oral therapies such as tetracyclines, folate inhibitors, quinolones, rifamycins and macrolides are usually in combination with topical therapy.[3]
Eradication of MRSA colonizers by mupirocin can bring down the infection rate among patients drastically. However, unrestricted over-the-counter use, treatment of wounds and pressure sores, and routine use in peritoneal dialysis with mupirocin are especially strongly associated with resistance which has been reported worldwide, and prevalence of such resistance will be a major setback for future use of mupirocin.[4] The study was also carried out to screen HCWs and medical students for MRSA and to know the susceptibility of mupirocin in this group. The results of the study will benefit the medical fraternity and society as whole by knowing the rate of MRSA prevalence and if any mupirocin resistance is present in our hospital. Hence, this study was conducted to know the prevalence of MRSA and MRCoNS from nasal swab of students and HCWs and also to determine the rates of high level and low level of mupirocin resistance in MRSA and MRCoNS spp. by disc diffusion.
Materials and Methods
A prospective, cross-sectional study was carried out in the Department of Microbiology of a tertiary care teaching hospital for 4 months from March 2015 to June 2015, after obtaining approval from the Institutional Ethics Committee (FMMC/FMIEC/2177/2015). Written informed consent was obtained from all the participants willing to participate in the study. A total of 432 students, nursing staff, doctors and house-keeping staff participated in the study. The demographic data including work profile and medical history were recorded. Only those persons who have entered hospital set-up for more than 1 year were included in the study. Persons with upper respiratory tract infections were excluded from the study.
Nasal swabs prewetted with sterile saline were collected from the vestibule of the anterior nares of both the nostrils and immediately placed back on the screw cap polypropylene tubes. These nasal swabs were streaked on Mueller-Hinton agar and kept for incubation at 37°C for 24 h. Identification of S. aureus was done by standard microbiological procedures. For detection of MRSA and MRCoNS, the isolates were tested for antimicrobial susceptibility testing by modified Kirby-Bauer disc diffusion method on Mueller-Hinton agar plates. Cefoxitin disc (30 μg) showing zone size of <22 mm was considered to be MRSA in case of tube coagulase-positive Staphylococcus, <25 mm zone size was considered MRCoNS in case of tube coagulase-negative Staphylococcus (CoNS) and zone size of <10 mm for bacitracin disc (0.04 units) was considered resistant.
The MRSA and MRCoNS isolates were then tested for mupirocin resistance, which was done by Kirby-Bauer disc diffusion method using 5 μg and 200 μg mupirocin discs to determine low- and high-level resistance.
Criteria of zone diameter breakpoints for susceptible and resistant isolates were set at >14 and <13 mm, respectively.[5] Three different phenotypes:
-
Mupirocin susceptible – zone diameter of ≥14 mm for both 5 μg and 200 μg discs
-
Low-level resistance – zone diameter of <14 mm in the 5 μg disc but ≥14 mm in the 200 μg disc
-
High-level resistance – isolates with zone diameter <14 mm for both 5 μg and 200 μg.
Statistical analysis
All the data were analyzed statistically using Chi-square test to calculate significant levels.
P < 0.05 was considered statistically significant.
Results
Out of the 432 who participated in the study, majority were staff nurses followed by students, doctors and house-keeping staff. Figure 1 shows mupirocin susceptibility.
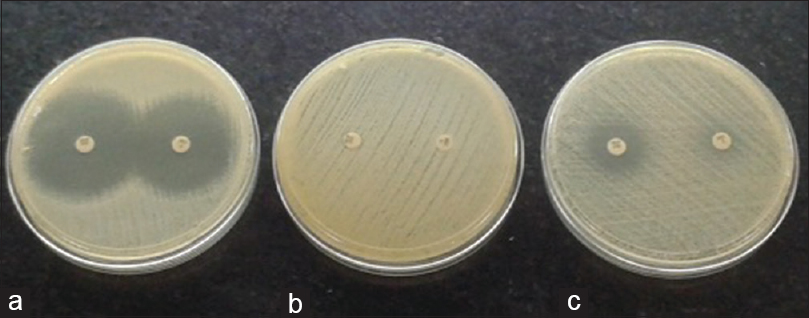
- (a) Mupirocin susceptible – for both 5 μg and 200 μg disc, (b) high-level resistance – for both 5 μg and 200 μg disc, (c) low- level resistance – resistance in the 5 μg disc but susceptible in the 200 μg disc
The category, culture results and isolates isolated are shown in Table 1. Predominant among 432 isolates, 24 (5.55%) were MRSA and 104 (24.07%) were MRCoNS.

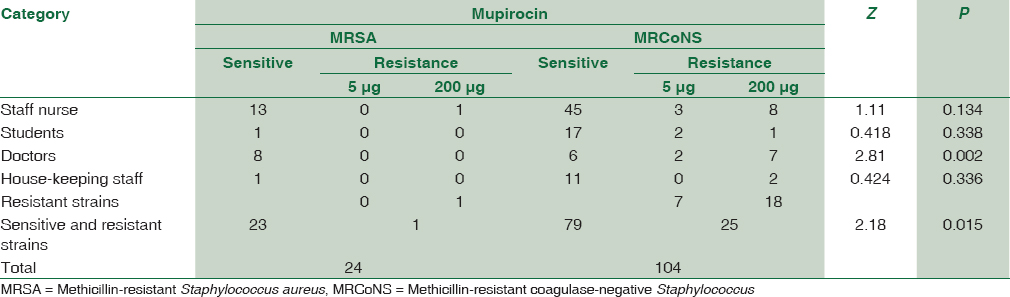
The MRSA and MRCoNS isolates tested for mupirocin resistance and significance of the study are shown in Table 2. Among 24 isolates of MRSA, only one showed high-level resistance to mupirocin, whereas out of 104 isolates of MRCoNS, 25 were resistant to mupirocin with 7 (28%) MupL and 18 (72%) MupH.
Discussion
In our study of 432 HCWs, we found that CoNS (n = 292) was more common than S. aureus (n = 129) of which 5.55% (n = 24) were MRSA and 24.07% (n = 104) were MRCoNS. The MRSA and MRCoNS tested for MupH and MupL showed only 4.16% (n = 1) to have high-level resistance among the MRSA isolates while resistance among MRCoNS was higher at 6.7% (n = 7) for low-level resistance and 17.30% (n = 18) for high-level resistance among the MRCoNS isolates.
Previous studies have shown that nasal carriers of MRSA are prevalent in HCWs. In a study by Golia et al., they revealed that HCWs were potential colonizers of MRSA and they found 13.37% in their study group.[6] In a study by Rongpharpi et al., they also found that the prevalence of S. aureus nasal carriers does exist with MRSA being reported at 11.43% and was higher among male HCWs.[7] Both the studies have reported a higher prevalence of MRSA than our study (5.5%) which may be due to the fact that hospital infection control committee of our hospital performs screening for MRSA in intensive care units and stringent measures are taken.
A study related to mupirocin resistance in HCWs in our country was previously studied by Kaur et al., where 140 HCWs were randomly selected. They isolated 38 S. aureus and 73 CoNS. Twenty MRSA and 34 MRCoNS were identified, of which two MRSA and five MRCoNS were mupirocin resistance.[8] Another study by Agarwal et al. reported 28 out of 200 HCWs showing nasal carriage of MRSA and mupirocin resistance was seen in four of them, of which three isolates were MupH and one was MupL.[9]
However, most of the studies done in our country are related to clinical isolates of S. aureus and its resistance to mupirocin. Chaturvedi et al. in their study showed that both high and low levels of mupirocin resistance MRSA were observed in patient population.[10] Oommen et al. also found that high-level mupirocin resistance was prevalent in clinical isolates.[11] Singh et al. in their study also reported presence of mupirocin resistant in MRSA isolates.[12]
We noticed that both low- and high-level resistance of mupirocin to MRCoNS is a disturbing fact as MRCoNS are known to cause hospital infections. Most of these isolates are found in staff nurses and doctors. We recommend strict vigilance by the Hospital Infection Control Committee to control this problem before it becomes a major health problem.
Conclusion
In our study, we conclude that MRSA colonization which may serve as a source of infection and mupirocin resistance is present among HCWs in our hospital setting though at a lower level when compared to other studies.
We suggest that screening for MRSA in HCWs should be performed for all whether working in Intensive Care Units or not, with susceptibility testing for mupirocin and if detected, alternative treatment should be used which will result in appropriate use of this antibiotic for decolonization.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Gram positive cocci. Staphylococci and related gram positive cocci. In: Koneman's Color Atlas and Textbook of Diagnostic Microbiology (6th ed). Baltimore: Lippincott Williams and Wilkins; 2006. p. :624-37.
- [Google Scholar]
- The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5:751-62.
- [Google Scholar]
- Does the nose know? An update on MRSA decolonization strategies. Curr Infect Dis Rep. 2013;15:455-64.
- [Google Scholar]
- Interpretive criteria for testing susceptibility of staphylococci to mupirocin. Antimicrob Agents Chemother. 1997;41:1137-9.
- [Google Scholar]
- A study of nasal carriage of MRSA among the health care workers of a tertiary care hospital, Bangalore. Int J Basic Appl Med Sci. 2013;3:3-7.
- [Google Scholar]
- The prevalence of nasal carriage of Staphylococcus aureus among healthcare workers at a tertiary care hospital in Assam with special reference to MRSA. J Clin Diagn Res. 2013;7:257-60.
- [Google Scholar]
- Mupirocin resistance in nasal carriage of Staphylococcus aureus among healthcare workers of a tertiary care rural hospital. Indian J Crit Care Med. 2014;18:716-21.
- [Google Scholar]
- Nasal carriage of methicillin- and mupirocin-resistant S. aureus among health care workers in a tertiary care hospital. J Res Pharm Pract. 2015;4:182-6.
- [Google Scholar]
- Prevalence of mupirocin resistant Staphylococcus aureus isolates among patients admitted to a tertiary care hospital. N Am J Med Sci. 2014;6:403-7.
- [Google Scholar]
- Mupirocin resistance in clinical isolates of staphylococci in a tertiary care centre in South India. Indian J Med Microbiol. 2010;28:372-5.
- [Google Scholar]
- Mupirocin resistance in clinical isolates of Staphylococcus aureus in a tertiary care hospital set up in North India. Int J Med Res Health Sci. 2013;2:840-7.
- [Google Scholar]





