Translate this page into:
Seropositivity of hepatitis A and E viruses in patients attending a tertiary care center in central India
*Corresponding author: Nagaraj Perumal, State Virology Laboratory, Gandhi Medical College, Bhopal, Madhya Pradesh, India. micronaga07@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Jain RK, Shrivastava RK, Jain SK, Chaurasia D, Jain A, Ahirwar KK, et al. Seropositivity of hepatitis A and E viruses in patients attending a tertiary care center in central India. J Lab Physicians. 2024;16:454-60. doi: 10.25259/JLP_97_2024
Abstract
Objectives:
Hepatitis A virus (HAV) and hepatitis E virus (HEV) infections are significant global health concerns that contribute to acute viral hepatitis. This study aimed to investigate the prevalence of HAV, HEV, and co-infections in a tertiary care hospital setting in central India.
Materials and Methods:
This retrospective observational study analyzed 987 clinical specimens collected from suspected acute viral hepatitis cases over 5 years (2019–2023). Commercially available enzyme-linked immunosorbent assay kits were used to detect HAV and HEV immunoglobulin M antibodies.
Statistical analysis:
Demographic data and clinical information were collected and analyzed using Chi-square tests. P < 0.05 was considered statistically significant, indicating a significant association between the variables under investigation.
Results:
Overall, 32.72% of patients were seropositive for either HAV, HEV, or both. The prevalence of HAV was 22.9%, HEV was 9.83%, and co-infection was 3.24%. HAV infection was more prevalent in children (0–14 years), whereas HEV was more prevalent in adults. Both HAV and HEV infections were associated with elevated liver function markers, with the highest levels observed in co-infected cases. The monsoon season had the highest number of cases.
Conclusions:
This study revealed a substantial burden of HAV, HEV, and co-infections in central India. The observed sex—and age-specific prevalence patterns warrant further investigation. Effective public health strategies addressing sanitation, hygiene practices, and HAV vaccination programs are crucial to reducing the disease burden.
Keywords
Hepatitis A virus
Hepatitis E virus
Hepatitis A virus-hepatitis E virus co-infection
Acute viral hepatitis
Seroprevalence
INTRODUCTION
Hepatitis A virus (HAV) and hepatitis E virus (HEV) infections represent a critical global health concern, ranking as the primary etiological agents of acute viral hepatitis. Characterized by a self-limiting course in most cases, these infections can nonetheless lead to significant morbidity.[1] Transmission of both HAV and HEV predominantly occurs through the fecal-oral route through ingestion of contaminated food or water. The burden of HAV and HEV prevalence disproportionately affects developing nations, where inadequate sanitation, limited access to clean drinking water, and poor hygiene practices create fertile ground for viral transmission.[2] Alarmingly, on a global scale, an estimated 1.4 million new HAV cases and a staggering 20 million new HEV cases emerge annually. Furthermore, HEV infection tragically claimed an estimated 44,000 lives globally in 2015 alone.[3]
HAV is a non-enveloped, single-stranded, and positive-sense RNA virus classified within the Picornaviridae family, specifically the Hepatovirus genus.[4,5] HAV infection, unlike its counterparts hepatitis B and C, does not establish a chronic carrier state within the host. The acute illness it incurs manifests with a constellation of symptoms, including fever, abdominal discomfort, diarrhea, darkened urine, anorexia, nausea, vomiting, and jaundice. In rare cases, fulminant hepatitis, characterized by severe liver dysfunction, can develop.[6,7] Fortunately, HAV infection is vaccine-preventable. In addition, natural infection frequently confers lifelong immunity, with seropositivity rates exceeding 80% in children by adulthood.[8] HAV exhibits a preference for the pediatric population, with a higher prevalence observed in children compared to adults.[9]
HEV, similar to HAV, is a single-stranded, positive-sense RNA virus. However, its taxonomic classification falls within the Hepeviridae family and the Orthohepevirus genus, distinct from HAV.[10] HEV infection, mirroring HAV, results in acute viral hepatitis. However, HEV poses a significant threat to pregnant women, potentially progressing to fulminant hepatitis and even mortality. HEV demonstrates a preference for adult populations compared to children and often follows both sporadic and epidemic patterns of incidence.[11-13]
HAV and HEV constitute a substantial public health burden in India, contributing significantly to the incidence of acute viral hepatitis. Estimates suggest that HAV infection is responsible for 10–30% of acute hepatitis cases, with a concerning 5–15% of these cases progressing to acute liver failure (ALF). HEV infection presents an even greater threat, potentially causing 10–40% of acute viral hepatitis cases and with a staggering 15–45% developing ALF.[14] Co-infections with both HAV and HEV, although less frequent, can lead to severe complications and a significantly increased risk of ALF, resulting in higher mortality rates among both children and adults.[15]
Despite the established burden of HAV and HEV infections in India, a paucity of data hinders a comprehensive understanding of their exact prevalence. Hence, this study is designed as a retrospective observational investigation to address the critical lack of data on HAV and HEV prevalence in central India. This will ultimately reduce the morbidity and mortality associated with these viral infections.
MATERIALS AND METHODS
Study design and sample collection
A retrospective observational study was conducted at a tertiary care hospital in central India. The study aimed to assess the prevalence of HAV, HEV, and co-infections among patients presenting with clinical features suggestive of acute viral hepatitis, such as jaundice, fever, abdominal pain, vomiting, dark urine, hepatomegaly, nausea, and diarrhea. The study analyzed the clinical samples collected over 5 years from January 2019 to December 2023. Only specimens meeting inclusion criteria were considered: those exhibiting symptoms suggestive of acute viral hepatitis and sufficient volume and quality. The study protocol was reviewed and approved by the Institutional Ethics Committee of Gandhi Medical College, Bhopal, Madhya Pradesh, India, vide letter no. 30690/MC/IEC/2022.
Serological investigations
Blood samples received at the laboratory underwent processing for serum separation. Briefly, samples were allowed to clot at room temperature and then centrifuged at 1500 rpm for 10 min. The separated serum was stored at 2–8°C for further analysis. To determine the presence of acute HAV and HEV infections, commercially available enzyme-linked immunosorbent assay (ELISA) kits anti-HAV immunoglobulin M (IgM) (DIA PRO Diagnostic Bioprobe Srt, Italy) and anti-HEV IgM (DIA PRO Diagnostic Bioprobe Srt, Italy) were employed. Each ELISA test incorporated positive and negative controls to ensure assay accuracy. All procedures were performed strictly following the manufacturer’s instructions.
Statistical analysis
Clinical data, demographic information, and other laboratory test results were collected and analyzed. The distribution of seronegative patients (absence of detectable antibodies) and seropositive (presence of detectable antibodies) for HAV and HEV was compared using a Chi-square test with Yates’ correction. P < 0.05 was considered statistically significant, indicating that the observed differences in seropositivity distribution between HAV and HEV groups were unlikely to be due to chance.
RESULTS
Patient demographics and seropositivity rates
A total of 987 clinical specimens were obtained from suspected acute viral hepatitis over 5 years (January 2019–December 2023). The study population comprised 621 (62.9%) males and 366 (37.1%) females. Urban residency (536, 54.3%) was more frequent compared to rural residency (451, 45.7%). The mean age of the study group was 26.84 years (±19.96 standard deviation [SD]), with males having a slightly higher mean age (28.4 years ± 20.1 SD) compared to females (24.2 years ± 19.5 SD).
Out of the 987, 323 (32.72%) were seropositive for either HAV, HEV, or both viruses. Among the seropositive cases, males (195, 60.4%) were more prevalent than females (128, 39.6%). Among these seropositive individuals, 226 (22.9%) were positive for HAV antibodies, 97 (9.83%) were positive for HEV antibodies, and 32 (3.24%) were co-infected with both HAV and HEV [Table 1].
| Characteristics | Total case | Total positive | HAV+ | HEV+ | HAV-HEV co-infection |
|---|---|---|---|---|---|
| Cases (n, %) | 987 (100) | 323 (32.72) | 226 (22.9) | 97 (9.83) | 32 (3.24) |
| Residency | |||||
| Urban (n, %) | 536 (54.3) | 220 (68.1) | 177 (78.3) | 43 (44.3) | 24 (75) |
| Rural (n, %) | 451 (45.7) | 103 (31.9) | 49 (21.7) | 54 (55.7) | 8 (25) |
| Gender | |||||
| Male (n, %) | 621 (62.9) | 195 (60.4) | 131 (58) | 64 (66) | 18 (56.25) |
| Female (n, %) | 366 (37.1) | 128 (39.6) | 95 (42) | 33 (34) | 14 (43.75) |
| χ2 test value | 43.7301 | 8.8975 | 3.521 | 6.0498 | 0.1304 |
| P-value | <0.00001 | 0.0028 | 0.06 | 0.014 | 0.718 |
HAV: Hepatitis A virus, HEV: Hepatitis E virus
Sex-based distribution of seropositivity
The prevalence of HAV infection was higher in males (131, 58%) compared to females (95, 42%). Conversely, the prevalence of HEV infection was significantly higher in males (64, 66%) compared to females (33, 34%). This trend continued for HAV-HEV co-infection, with a higher prevalence observed in males (18, 56.25%) compared to females (14, 43.75%) [Table 1].
Age-based seropositivity
HAV infection exhibited a significantly higher prevalence (n = 169, 74.8%) in the pediatric population (0–14 years) compared to adults (n = 57, 25.2%). Conversely, HEV infection demonstrated a reverse trend, with a significantly greater prevalence in adults (n = 69, 71.1%) compared to children (n = 28, 28.9%). Interestingly, HAV-HEV co-infection displayed a similar pattern to HAV, with a higher prevalence in children (n = 21, 65.6%), although the difference compared to adults (n = 11, 34.4%) was not statistically significant [Table 2]. The graphical representation in Figure 1 effectively depicts the age-specific distribution of HAV and HEV seropositivity.
| Cases | Seropositive | Seropositive group n (%) | χ2 test value | P-value | |
|---|---|---|---|---|---|
| Pediatric | Adult | ||||
| HAV | 226 | 169 (74.8) | 57 (25.2) | 37.0289 | <0.00001 |
| HEV | 97 | 28 (28.9) | 69 (71.1) | 10.9405 | 0.0009 |
| Co-infection | 32 | 21 (65.6) | 11 (34.4) | 1.5217 | 0.2173 |
HAV: Hepatitis A virus, HEV: Hepatitis E virus, Bold values: Highly significant
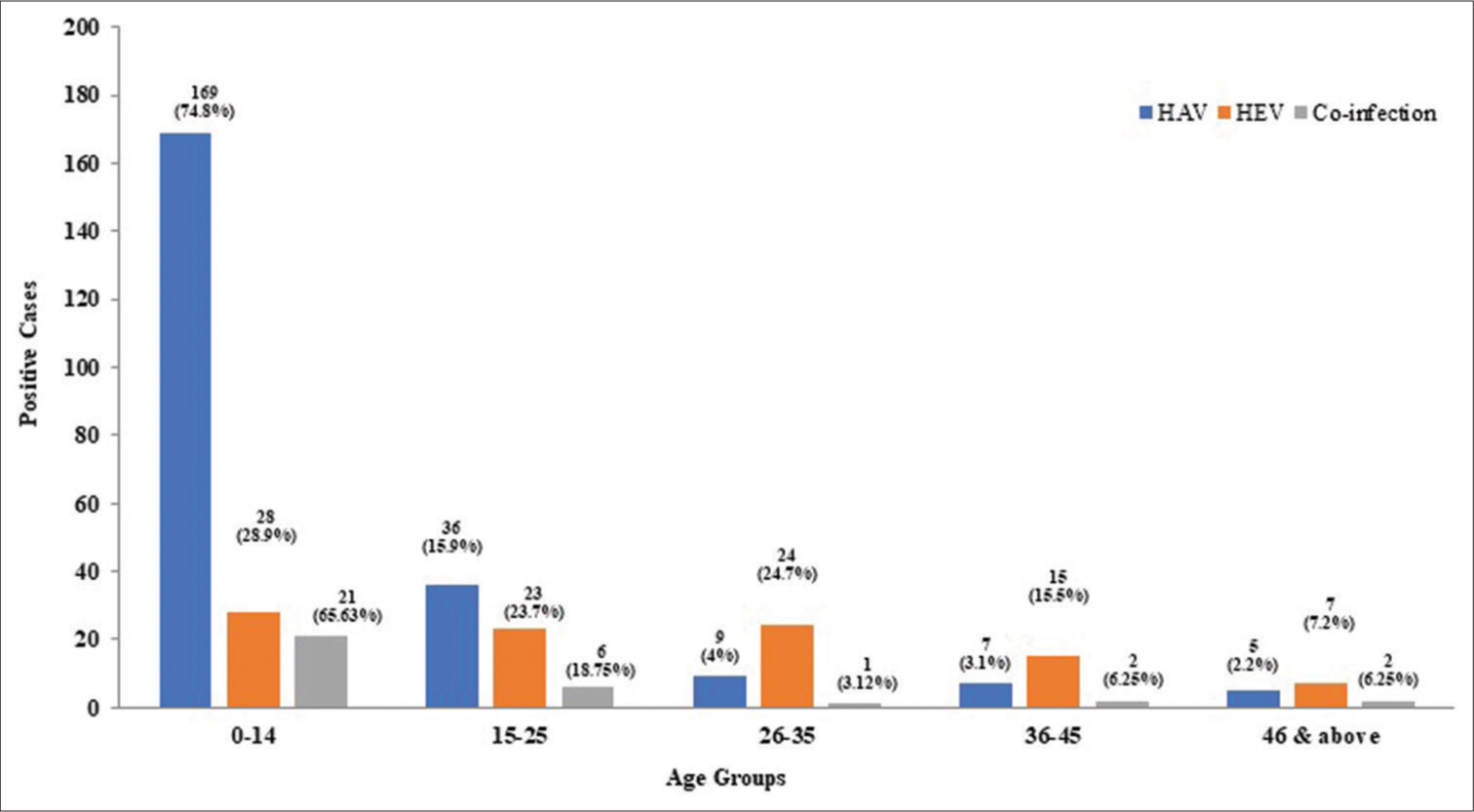
- Age-specific seropositivity of hepatitis A virus (HAV), hepatitis E virus (HEV), and co-infection.
Liver function biomarkers and seropositivity
Both HAV and HEV infections were associated with elevated levels of total serum bilirubin and alanine aminotransferase (ALT), indicating liver dysfunction. Notably, the highest bilirubin and ALT levels were observed in cases of HAVHEV co-infection. Table 3 summarizes these findings.
| Total case | HAV+ | HEV+ | HAV-HEV co-infection | |
|---|---|---|---|---|
| Serum bilirubin (mg/dL) | 5.51±5.87 | 7.38±6.65 | 7.78±7.1 | 8.34±6.6 |
| ALT (SGPT) (Unit/L) | 631.4±527.47 | 692.2±498.17 | 797.8±647.9 | 826±567.6 |
HAV: Hepatitis A virus, HEV: Hepatitis E virus, ALT: Alanine transaminase, SGPT: Serum glutamic-pyruvic transaminase
Seasonal distribution of seropositivity
The monthly trend of HAV and HEV prevalence, as depicted in Figure 2, demonstrates cases throughout all seasons. The monsoon season (July to October) witnessed the highest number of cases (n = 151), whereas the summer season (March to June) had the lowest number of cases (n = 57).
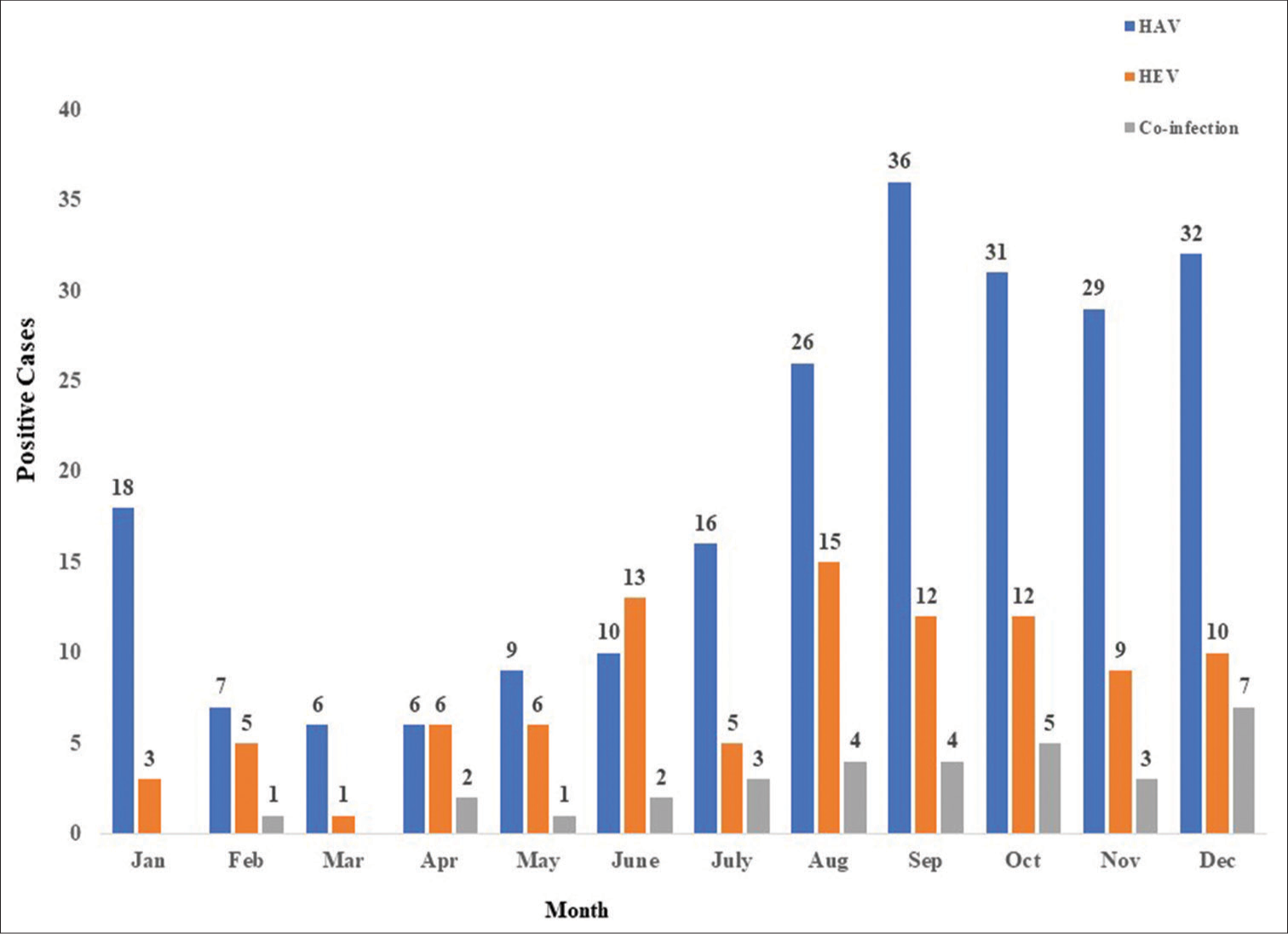
- Month-wise prevalence of hepatitis A virus (HAV), hepatitis E virus (HEV), and HAV+HEV co-infection.
DISCUSSION
This retrospective study conducted over 5 years at a tertiary care hospital in central India sheds light on the prevalence of HAV and HEV infections among patients presenting with clinical features suggestive of acute viral hepatitis. Globally, HAV and HEV are recognized as leading causes of acute viral hepatitis, often presenting as sporadic or epidemic outbreaks.[1] Both infections are typically self-limiting, although severe cases, particularly in pregnant women, can lead to mortality.[12,13] In India, the true nationwide prevalence of HAV and HEV remains elusive due to the absence of a robust national surveillance system. The sheer population size of India also presents challenges in real-time data collection for infectious diseases. However, several scattered studies from different states of India have reported varying prevalence of HAV and HEV.[16-25]
Our findings revealed a higher prevalence of HAV (22.9%) compared to HEV (9.83%) among the study population. This aligns with the range reported in prior studies conducted across various regions of India, where HAV prevalence varied between 2.1% and 27%, and HEV prevalence ranged from 8.5% to 68.42%[14-22,25] [Table 4]. These variations in prevalence across regions can likely be attributed to differences in local water and food contamination patterns, as both HAV and HEV primarily spread through the fecal-oral route through the ingestion of contaminated water or food.[2]
| Other study | City/State | Total cases | Prevalence (%) | |
|---|---|---|---|---|
| HAV | HEV | |||
| Jain et al., 2013[16] | Lucknow, Uttar Pradesh | 267 | 26.96 | 17.97 |
| Joon et al., 2015[17] | Mangalore, Karnataka | 958 | 19.31 | 10.54 |
| Agrawal et al., 2016[18] | New Delhi, Delhi | 475 | 9.47 | 23.36 |
| Rawat et al., 2019[7] | Rohtak, Haryana | 307 | 31.1 | 44.9 |
| Samaddar et al., 2019[20] | Mumbai, Maharashtra | 675 | 6.96 | 9.63 |
| Barde et al., 2019[22] | Jabalpur, Madhya Pradesh | 1901 | 5.1 | 13.7 |
| Bansal et al., 2022[1] | Srinagar, Uttarakhand | 5319 | 16.9 | 14.9 |
| Palewar et al., 2022[24] | Pune, Maharashtra | 1807 | 6.7 | 8.5 |
| Kaur et al., 2017[25] | Amritsar, Punjab | 95 | 2.1 | 68.42 |
HAV: Hepatitis A virus, HEV: Hepatitis E virus
In this study, the male population has a higher rate (HAV-58% and HEV-66%) of infection in comparison with females (HAV-42% and HEV-34%). While the reasons for this disparity require further investigation, it is possible that social and behavioral factors in India, where males may participate more in outdoor activities and social gatherings, contribute to increased exposure risk.[20] Both HAV and HEV infections typically resolve spontaneously; however, in rare instances, they may lead to fulminant liver failure, and the study by Singh et al.[21] underscores the importance of recognizing the risk of fulminant hepatic failure, especially in vulnerable populations such as pregnant women. Management primarily involves supportive care and ensuring adequate rest. Since both infections are spread enterically through contaminated water or food, preventive efforts should focus on improved sanitation, hygiene practices, and public education campaigns promoting safe food and water consumption.[17]
The study revealed distinct age-related patterns for HAV and HEV infections. HAV exhibited a significantly higher prevalence (74.8%) in the pediatric population (0–14 years), with a decrease observed in older age groups (25.2% in >15 years). This finding aligns with the common belief that HAV primarily infects children. Conversely, HEV infection demonstrated a peak prevalence in the pediatric group (28.9%), followed by similar prevalence in the 15–25 years and 26–35 years age groups. Childhood HAV infection is typically asymptomatic and confers lifelong immunity. However, the study observed a trend of HAV infection occurring at a later age (adolescence and early adulthood) in the studied population. This might be attributed to improvements in living standards and socioeconomic conditions, leading to decreased exposure in early childhood.
The study observed a co-infection prevalence of 3.24%, with a slightly higher prevalence in children compared to adults. While co-infection generally does not significantly impact disease course, it underscores the importance of timely diagnosis using serological and molecular tests, especially in children and adults, to ensure appropriate management and prevent potential complications like ALF.[26-28]
The study observed elevated levels of total serum bilirubin and ALT in all cases, with the highest levels occurring in individuals co-infected with HAV and HEV. These findings are consistent with normal ranges (bilirubin: 0.2–1.2 mg/dL; ALT: 7–56 U/L) and align with previous research.[20,29,30] Elevated liver enzymes are indicative of liver dysfunction, and the more pronounced elevations observed in co-infection cases suggest a potential link between dual infection and increased disease severity. This finding underscores the importance of identifying co-infection promptly to ensure appropriate management and potentially reduce the risk of complications such as ALF and hepatic encephalopathy.
The study observed that HAV and HEV cases occur throughout the year, with a seasonal peak during the monsoon season (July to October). This coincides with the period when heavy rainfall can increase the risk of sewage contamination of drinking water sources.[31] The study also identified a secondary peak in winter, suggesting potential additional factors influencing transmission beyond just monsoon rains. Conversely, the summer season witnessed a relatively lower number of cases. This seasonal trend highlights the potential for targeted public health interventions to reduce HAV and HEV transmission. Pre-monsoon and monsoon season campaigns led by public health departments can play a crucial role in raising awareness about safe water consumption, proper sanitation practices, and hand hygiene.
Fecal-orally transmitted viral hepatitis, including HAV and HEV, represents a significant public health concern worldwide. Outbreaks are often linked to inadequate sanitation infrastructure, particularly open defecation practices that contaminate water sources. Unsafe drinking water, improper sewage disposal, and poor hygiene practices all contribute to the spread of these viruses.
The present study has some inherent limitations due to its retrospective design. Retrospective data collection may be susceptible to under-reporting of cases, and outbreak events might not have been captured comprehensively. In addition, information regarding the pregnancy status of females in the study population was not available. This limits our understanding of HEV prevalence among pregnant women, a high-risk group for severe complications. Finally, due to financial constraints, genotype analysis of the HAV and HEV strains could not be performed. Genotype data could provide valuable insights into the origin and transmission patterns of these viruses in the study population.
CONCLUSIONS
This study investigated the prevalence of HAV and HEV infections in a tertiary care hospital setting. The findings reveal a higher seroprevalence of HAV compared to HEV, suggesting a potential benefit of incorporating HAV screening in the diagnostic workup for suspected cases, particularly among children. In addition, the observed co-infection rate underscores the importance of timely diagnosis using serological and molecular tests. These findings highlight the need for comprehensive public health strategies to reduce HAV and HEV transmission. Effective strategies likely involve collaboration between public health and healthcare systems to (i) Improve sanitation infrastructure, (ii) promote proper hygiene practices, (iii) implement HAV vaccination programs, and (iv) maintain ongoing surveillance of HAV and HEV infections. By addressing these critical areas, healthcare systems and public health agencies can work toward reducing the morbidity, mortality, and economic burden associated with HAV and HEV infections.
Ethical approval
The research/study approved by the Institutional Review Board at The Institutional Ethics Committee of Gandhi Medical College, Bhopal, number 30690/MC/IEC/2022, dated 04th August 2022.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Seroprevalence of hepatitis A and hepatitis E in patients at a teaching hospital of northern India over a period of 8 years. J Family Med Prim Care. 2022;11:567-72.
- [CrossRef] [Google Scholar]
- An outbreak of hepatitis A in a French day-care center and efforts to combat it. Eur J Epidemiol. 1997;13:139-44.
- [CrossRef] [Google Scholar]
- Hepatitis. Available from: https://www.emro.who.int/health-topics/hepatitis/introduction.html [Last accessed on 2024 Feb 19]
- [Google Scholar]
- Hepevirus In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, eds. Virus taxonomy: Eighth report of the International Committee on Taxonomy of Viruses. London, United Kingdom: Elsevier/Academic Press; 2004. p. :851-5.
- [Google Scholar]
- Hepatitis A. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-a [Last accessed on 2024 Feb 19]
- [Google Scholar]
- Prevalence of hepatitis A virus and hepatitis E virus in the patients presenting with acute viral hepatitis in Rohtak, Haryana, India. Int J Res Med Sci. 2019;7:1792-5.
- [CrossRef] [Google Scholar]
- Epidemiology of hepatitis A and hepatitis E based on laboratory surveillance data-India, 2014-2017. Am J Trop Med Hyg. 2018;99:1058-61.
- [CrossRef] [Google Scholar]
- Genetic variability of hepatitis A virus. J Gen Virol. 2003;84:3191-201.
- [CrossRef] [Google Scholar]
- Hepatitis E virus genome structure and replication strategy. Cold Spring Harb Perspect Med. 2019;9:a031724.
- [CrossRef] [Google Scholar]
- Evidence of recombination between divergent Hepatitis E virus. J Virol. 2005;79:9305-14.
- [CrossRef] [Google Scholar]
- Hepatitis A and E. Available from: https://www.hopkinsmedicine.org/health/conditions-and-diseases/hepatitis/hepatitis-a [Last accessed on 2022 Nov 23]
- [Google Scholar]
- Investigation of a hepatitis A outbreak from Shimla, Himachal Pradesh. Indian J Med Res. 2009;130:179-84.
- [Google Scholar]
- National viral hepatitis control program-operational guidelines 2018 issued by Ministry of Health and Family Welfare. Government of India. Available from: https://www.nvhcp.mohfw.gov.in/common_libs/diagnosis-management-viralhepatitis.pdf [Last accessed on 2024 Mar 23]
- [Google Scholar]
- Viral hepatitis surveillance India, 2011-2013. MMWR Morb Mortal Wkly Rep. 2015;64:758-62.
- [CrossRef] [Google Scholar]
- Prevalence of hepatitis A virus, hepatitis B virus, hepatitis C virus, hepatitis D virus and hepatitis E virus as causes of acute viral hepatitis in North India: A hospital based study. Indian J Med Microbiol. 2013;31:261-5.
- [CrossRef] [Google Scholar]
- Prevalence of Hepatitis A virus (HAV) and Hepatitis E virus (HEV) in the patients presenting with acute viral hepatitis. Indian J Med Microbiol. 2015;33:102-5.
- [CrossRef] [Google Scholar]
- A study of seroprevalence and co-infection of hepatitis A and hepatitis E viruses in sporadic cases in an endemic area. J Med Sci Health. 2016;2:1-5.
- [CrossRef] [Google Scholar]
- Epidemiological study of hepatitis A virus and hepatitis E virus infection in patients presenting with acute viral hepatitis. Int J Curr Microbiol App Sci. 2018;7:899-904.
- [CrossRef] [Google Scholar]
- Infectious hepatitis: A 3-year retrospective study at a tertiary care hospital in India. Indian J Med Microbiol. 2019;37:230-4.
- [CrossRef] [Google Scholar]
- Outcome of hepatitis E virus infection in Indian pregnant women admitted to a tertiary care hospital. Indian J Med Res. 2001;113:35-9.
- [Google Scholar]
- Viral hepatitis among acute hepatitis patients attending tertiary care hospital in central India. Virus Dis. 2019;30:367-72.
- [CrossRef] [Google Scholar]
- Prevalence of enterically transmitted hepatitis viruses in patients attending a tertiary-care hospital in South India. Indian J Pathol Microbiol. 2000;43:433-6.
- [Google Scholar]
- Prevalence of hepatitis A virus (HAV) and hepatitis E virus (HEV) in patients presenting with acute viral hepatitis: A 3-year retrospective study at a tertiary care Hospital in Western India. J Family Med Prim Care. 2022;11:2437-41.
- [CrossRef] [Google Scholar]
- Hepatitis E virus: A leading cause of waterborne viral hepatitis in Northwest Districts of Punjab, India. J Lab Physicians. 2017;9:121-4.
- [CrossRef] [Google Scholar]
- Outbreak of acute viral hepatitis due to hepatitis E virus in Hyderabad. Indian J Med Microbiol. 2007;25:378-82.
- [CrossRef] [Google Scholar]
- Acute viral hepatitis types E, A, and B singly and in combination in acute liver failure in children in north India. J Med Virol. 1996;48:215-21.
- [CrossRef] [Google Scholar]
- Seroprevalence of hepatitis A virus (HAV) and hepatitis E virus (HEV) co-infection in the patients presenting with acute viral hepatitis attending a tertiary care hospital in North India. J Community Dis. 2017;49:57-60.
- [CrossRef] [Google Scholar]
- Prevalence of hepatitis A virus and hepatitis E virus in the patients presenting with acute viral hepatitis at a tertiary care hospital Jaipur Rajasthan. N Niger J Clin Res. 2016;5:47-50.
- [CrossRef] [Google Scholar]
- A case of co infection of hepatitis A and E virus with hepatic encephalopathy. Korean J Med. 2011;80(Suppl 2):S1015.
- [Google Scholar]
- Predicting acute viral hepatitis serum markers (A and E) in patients with suspected acute viral hepatitis attending primary health care centers in Baghdad: A one year crosssectional study. Glob J Health Sci. 2012;4:172-83.
- [CrossRef] [Google Scholar]






