Translate this page into:
Serum Uric Acid Level in Relation to Severity of the Disease and Mortality of Critically Ill Patients
Address for correspondence: Dr. Hale Rashidian, E-mail: drhalerashidian@hotmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Aim:
This study was conducted to evaluate the validity of serum uric acid (UA) in prediction of mortality among patients in the emergency department.
Materials and Methods:
This is a prospective cohort study which was conducted during 2014. In this study, 120 critically ill patients who required Intensive Care Unit care services were included. For evaluation of severity of the disease, mortality in emergency department score (MEDS) in the first 24 h of admission, the requirement of using mechanical ventilation, taking vasopressor during the hospitalization time and severity of the disease based on MEDS score were measured. The patients were divided into two groups: Patients with serum UA level lower than 7.3 mg/dl and patients with serum UA level of equal or more than 7.3 mg/dl. For comparison of the mortality rate in groups, Chi-square and fisher exact tests were applied.
Results:
In patients, who needed mechanical ventilation, average of serum UA was 7.82 ± 2.82; however, in the patients who did not need mechanical ventilation this amount was 6.16 ± 2.7, a difference was statically significant. We found a statically meaningful difference between serum UA level with requiring mechanical ventilation and the predictive level of UA 6.95 ± 0.73 (F = 8.52; P ≤ 0.004). In the evaluation of MEDS, most patients with serum UA levels lower than 7.3 mg/dl had lower MEDS points (on average 4.6 ± 3.21) in compared to patients with serum UA level higher than 7.3 mg/dl (on average 12 ± 2.99). This difference was found to be statistically significant which indicates the patients whose serum UA was 7.3 mg/dl or higher, were at higher risk of mortality.
Conclusion:
The serum UA level in the 1st day of hospitalization of a critically ill patient is not an independent indicative factor in relation to mortality. High level of UA reveals critical status of the patient and requires mechanical ventilation.
Keywords
Critical ill patients
mortality
serum uric acid
INTRODUCTION
Uric acid (UA) is an end product of purine base metabolism and an antioxidant agent. Serum UA concentration is influenced by several factors such as overproduction, decreased glomerular filtration or renal hypoperfusion, enhanced tubular reabsorption or diminished elimination.[1] That is degraded by urate oxidase (urease) to allantoin, which is freely eliminated in urine.[2] Hyperuricemia is generally defined by UA levels >6.5 mg/dl or 7 mg/dl in men and >6 mg/dl in women.[3] The role of UA as an independent causative or potential risk factor of mortality is controversial in patients with kidney disease, hypertension, obesity, cardiovascular events, diabetes mellitus, ischemic stroke, and cancer disease.[456] It was shown that serum UA level changed in patients with more severe sepsis.[7] These data show the combined effect of several factors on UA. The changes in UA in the clinical setting and pathophysiological events are related to oxidative stress, and provide evidence of impaired plasma antioxidant capacity in severe sepsis.[8] A correlation has been found between serum UA level and inflammatory markers on population-based cohort studies.[9] Serum UA concentrations can be considered as a marker of severity in critically ill patients without craniocerebral trauma and especially in patients with meningococcal infection.[10] Changes in hypoxanthine, xanthine, UA concentrations, and oxygen transport parameters can be used to assess changes in the functioning of the microcirculatory bed. It has been established that an increased blood plasma level of hypoxanthine and xanthine may serve as an additional criterion of tissue hypoxia in critically ill surgical patients.[11] UA level during the 1st day of intensive critical care admission is not an independent risk of mortality in Pediatric Intensive Care Unit (ICU), although the presence of sepsis and high level of UA have been associated with poor outcome.[12] A number of scoring systems have been developed that circumvent the complexity and time constraints of traditional scoring systems developed to use in ICU setting.[13] The recent scorings include the MEDS score and the rapid emergency medicine score.[14] These scoring systems contain many items that should be checked in different times after admission, and sometimes are very time-consuming. We presumed that UA level at the early hours of admission could predict the mortality of the admitted patients as a simple single test. This study was conducted to evaluate the validity of serum UA in prediction of mortality in patients in the emergency department.
MATERIALS AND METHODS
This is a prospective cohort study which was conducted during June–December 2014 in observation unit of the emergency room of Imam Khomeini Hospital, Sari, Iran. The unit has four beds with intensive care facilities. In this study, 120 (above 18 years old) critically ill patients who required intensive care services were included. Patients who needed intensive care for the first time and maintained in the same condition for at least 24 h were included in the study and their blood samples were taken before initiation of any treatment. The exclusion criteria was; age under 18 years old, having trauma, acute coronary syndrome, myocardial infarction, hemodialysis, plasma creatinine of above three, any type of cancer, patients with hyperuricemia, and patients taking allopurinol.
Consent was taken after giving proper description of the process to the patients. This study was accepted and confirmed by the Ethics Committee of Mazandaran University of Medical Sciences. The epidemiological and clinical information of the patients was obtained by one physician and confirmed by another. UA level was measured by using the colorimetric uricase methods in only one selected laboratory of Imam Khomeini Hospital with the instrument Selectra E Device and ready to use UA TOOS commercial reagent kits obtained from UA Pars Azmoon Co, Iran. For evaluation of the severity of the disease, MEDS was used in the first 24 h after admission.[1415]
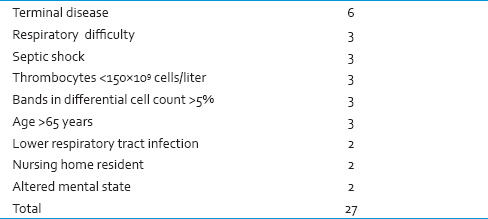
In this system, the scores range is between 0 and 27, with nine items and the scoring system are as follows: Respiratory distress (defined as having respiratory rate above 20/min or O2sat <90% in the normal room air or needing oxygenation with mask or experiencing apnea): Three points, Septic shock (clinical symptoms indicative of positive systemic inflammatory response syndrome and suspicious or confirmed infectious foci accompanied with resistance to primary fluid therapy hypotension.[16] Three points, platelet count <150,000: Three points, bandemia >5%: Three points, age >65 years: Three points, lower respiratory tract infection (defined as infiltration in chest X-ray or clinical evidence indicative of this diagnosis): Two points, needing in-home care nursing service: Two points, and any change in the level of awareness: This system included nine items, scores ranged 0-27. These scores explained as following: 0–4: Very low risk, 5–7: Low risk, 8–12: Moderate risk, 13–15: High risk, 15< very high risk. See scoring system in Table 1.
Mortality rate of the patients in the first 28 days of hospitalization was considered as the primary outcome. Furthermore, the requirement of using mechanical ventilation and taking vasopressor during the hospitalization time and severity of the disease based on MEDS score were measured.
Statistics
Finally, relationship of the serum UA level was evaluated by the cutoff point of 7.3 mg/dl for each outcome. Receiver operating characteristic (ROC) curve area was used for determination of the validity of UA level test in prediction of mortality. For comparison of the groups below 7.3 mg/dl UA group and above 7.3 mg/dl UA group, Chi-square and independent t-test used P < 0.05 were considered statistically significant.
RESULTS
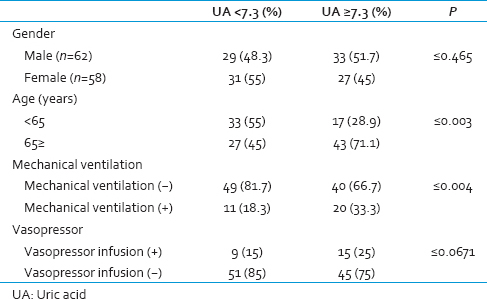
Sixty-two males (51.7%) and 58 females (48.3%) were included in the study. Of 120, 70 patients (58.3%) were older than 65 years old. The patients were divided into two groups: Patients with serum UA level of lower than 7.3 mg/dl and patients with serum UA level of equal or more than 7.3 mg/dl. Each group consisted of sixty patients: 29 (48.3%) males and 31 (51.7%) females in the serum UA <7.3 mg/dl group, 33 (55%) males and 27 (45%) females in the serum UA ≥7.3 mg/dl. No significant statistical differences were found according to the gender of patients in these groups. In general, the average age of the patients in the serum UA ≥7.3 mg/dl group was significantly higher.
During the study, 31 patients underwent mechanical ventilation, from which the number of patients with serum UA levels of higher than 7.3 mg/dl were more than other group, but this difference was not statically significant (P = 0.061). In patients who needed mechanical ventilation, an average of serum UA was 7.82 ± 2.82; however, in the patients who did not need mechanical ventilation this amount was 6.16 ± 2.7, the difference was statically significant [Table 2].
In the ROC analysis with the cutoff point of 6.95 ± 0.73 for UA level, the under the curve area of 0.667 (95% confidence interval [CI]: 0.561–0.774) with 71% sensitivity and 55.1% specificity for prediction of patient's need to use mechanical ventilation were taken into account (P ≤ 0.006) [Figure 1]. Therefore, UA level is related to patient's need to use mechanical ventilation.
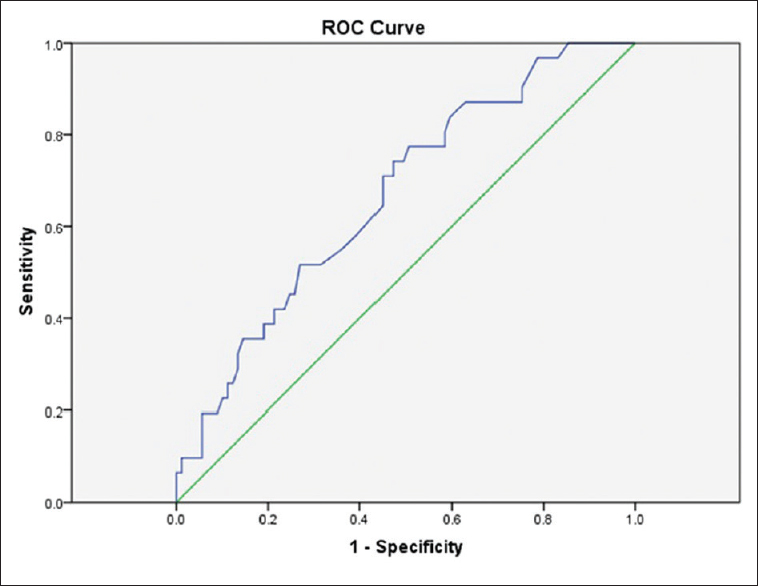
- Receiver operating characteristic curve for predicting the need of using ventilator according uric acid level cutoff point
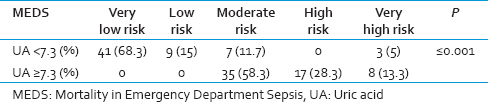
During this study, majority of patients who needed to take vasopressor medication had serum acid uric level of above 7.3 mg/dl. The mean serum UA level in the vasopressor taking patients was 7.55 ± 2.54. Out of 120 participants, 96 patients who did not take vasopressor, the mean serum UA level was 6.39 ± 2.84. There was no statistical significance found between vasopressor taking and nontaking patients. In the evaluation of MEDS, most patients having serum UA levels of lower than 7.3 mg/dl had lower MEDS points (on average 4.6 ± 3.21) in compared to patients with high serum UA levels (on average 12 ± 2.99). This difference was found to be statically significant which, indicates the patients with serum UA equal to 7.3 mg/dl or higher, were at higher risk of mortality [Table 3].
Twelve patients expired during the study, 9 of whom were older than 65 years old. Total mean serum UA level was 7.65 ± 3.25, although, in patients who did not survive during the study it was higher than survived patients (6.47 ± 2.76), this difference was not significant (P ≤ 0.171).
Furthermore, results showed that expiry of patients underwent mechanical ventilation and took vasopressor, were significantly higher than other patients [Table 4].
Using serum UA level cutoff point for predicting possibility of patients' mortality via ROC curve was not found to be significant (area under curve: 0.595 [CI: 0.422–0.770], P = 0.27) [Figure 2]. Therefore, serum UA level cutoff point could not predict possibility of patients' mortality in this study.
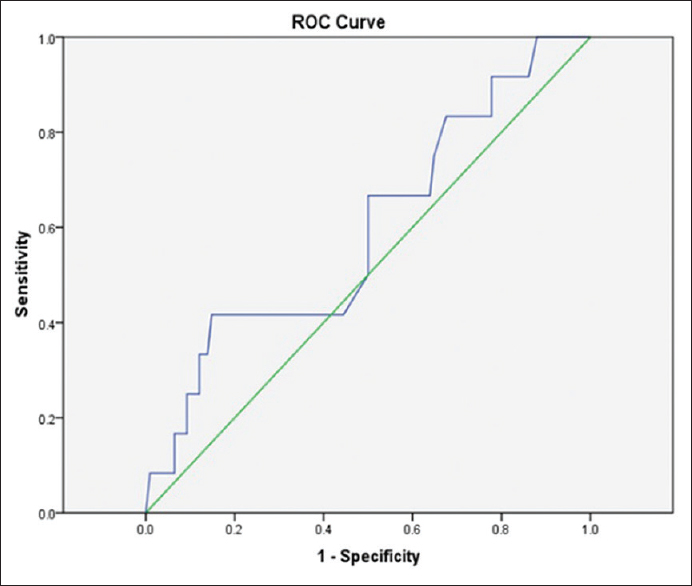
- Receiver operating characteristic curve for determination of serum uric acid cutoff point to predict mortality in patients
DISCUSSION
Based on the results of this study, there is no relationship between serum UA level and mortality rate of patients regardless of their underlying diseases. However, serum UA levels were meaningfully higher in patients who underwent mechanical ventilation. Using mechanical ventilation, infusion of Vasopressor and age of higher than 65 years, were independently associated with an increase in mortality rate. Furthermore, MEDS was directly associated with the serum UA level. UA level is an independent risk factor in the treatment of hypertension[17] and is also associated with the cardiovascular accidents.[18] UA level is considered as an index in early diagnosis of renal failure and patients with asymptomatic hyperuricemia are at higher risk of progression to end-stage renal disease.[19] Various factors effect on serum UA level and changes in UA level are related to oxidative stress in the clinical situation. These changes cause disturbance in the antioxidative capacity of plasma, especially in septic patients.[8] By increase in Acute Physiology and Chronic Health Evaluation (APACHE II) score, a special pattern is noted in plasma metabolism, and plasma UA amount increases progressively in patients with poor medical conditions.[20] In one study, it has been shown that the level of serum UA in the 1st day of admission in ICU was not related to the mortality rate; however, effects of mechanical ventilation and resistant shock were the main mortality factors regardless of serum UA level.[12] These research results are in agreement with our findings. However, in a study on fifty critically ill patients the level of serum UA was shown to be higher in nonsurvivor patients compared to the survivors.[21] The serum UA levels of 7 mg/dl or higher, will extend the hospitalization time of patients in ICU and also increases the time of using mechanical ventilation in patients.[22] The level of serum UA increases in respiratory disorders, especially if hypoxia and systemic inflammation exist. The Bartziokas et al. study, which was done on patients having respiratory diseases, revealed that serum UA level ≥6.9 mg/dl is an independent predicting factor in 1-month mortality of the patients. Moreover, duration of hospitalization in ICU and using mechanical ventilation are longer in these patients in 1 month.[23] One of the most important findings in our study was the relationship between needing to use the mechanical ventilation and the amount of serum UA; it means that serum UA level of 6.95 ± 0.73 and above has 71% sensitivity and 55.1% specificity in prediction of demanding mechanical ventilation. Patients with acute respiratory failure who needed mechanical ventilation had a higher amount of UA urea and mortality rate.[24] Similarly, in our study, the mortality rate was higher in patients who underwent mechanical ventilation and took Vasopressor. In the Hooman et al. study, needing to administer Vasopressor was shown to be an independent variable in poor outcome patients.[12] The level of serum UA has a direct relationship with APACHE II score, which means that patients with high APACHE II score have higher serum UA levels.[2125] Similarly, in our study there was a direct association between serum UA and MEDS; however, the mortality rate was not associated with MEDS. In this study, there were various factors such as dehydration, taking nephrotoxic drugs, severity of the underlying disease, instability of the vital signs, and medical complications during hospitalization (e.g., nosocomial infections) which can influence the outcome of the patients; therefore, these factors and incidents, when occurring during the hospitalization time can have an effect on the mortality of the patients the same way as the primary condition of the patients. Considering this issue and limitations of this study, the predictive value of serum UA in patients' mortality can be improved by measuring the serum UA several times, taking into account the medical complications during hospitalization and uniform sampling of the patients.
CONCLUSION
The serum UA level in the 1st day of hospitalization of a critically ill patient is not an independent indicative factor for mortality; however, age of above 65 years, requirement of mechanical ventilation and a shock which needs Vasopressor infusion, indicate a poor outcome for the patient regardless of his serum UA level. However, high level of UA reveals critical status of the patient and requires mechanical ventilation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Loss of urate oxidase activity in hominoids and its evolutionary implications. Mol Biol Evol. 2002;19:640-53.
- [Google Scholar]
- La hyperuricemia como factor de riesgo cardiovascular y renal. Dial Transplant. 2011;32:57-61.
- [Google Scholar]
- Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 2003;41:1183-90.
- [Google Scholar]
- Contribution of uric acid to cancer risk, recurrence, and mortality. Clin Transl Med. 2012;1:16.
- [Google Scholar]
- Review of hyperuricemia as new marker for metabolic syndrome. ISRN Rheumatol. 2014;2014:852954.
- [Google Scholar]
- Serum uric acid level, longitudinal blood pressure, renal function, and long-term mortality in treated hypertensive patients. Hypertension. 2013;62:105-11.
- [Google Scholar]
- Calculated total radical antioxidant potential compared in outcome groups of septic shock. Clin Intensive Care. 1999;10:7-12.
- [Google Scholar]
- Biochemical and clinical correlates of hypouricemia in surgical and critically ill patients. Clin Chem Lab Med. 2007;45:1207-10.
- [Google Scholar]
- Uric acid as a prognostic marker in critically ill patients. An Esp Pediatr. 2001;55:305-9.
- [Google Scholar]
- The concentration of end products of purine metabolism as possible signs of tissue hypoxia in critically ill surgical patients. Anesteziol Reanimatol. 1993;4:41-3.
- [Google Scholar]
- The value of serum uric Acid as a mortality prediction in critically ill children. Iran J Pediatr. 2010;20:323-9.
- [Google Scholar]
- Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754-8.
- [Google Scholar]
- Mortality in emergency department sepsis (MEDS) score: A prospectively derived and validated clinical prediction rule. Crit Care Med. 2003;31:670-5.
- [Google Scholar]
- Usefulness of severity scores in patients with suspected infection in the emergency department: A systematic review. J Emerg Med. 2012;42:379-91.
- [Google Scholar]
- Toward an epidemiology and natural history of SIRS (systemic inflammatory response syndrome) JAMA. 1992;268:3452-5.
- [Google Scholar]
- Uric acid as an independent risk factor in the treatment of hypertension. Lancet. 1998;352:670-1.
- [Google Scholar]
- Serum uric acid and cardiovascular events in successfully treated hypertensive patients. Hypertension. 1999;34:144-50.
- [Google Scholar]
- Significance of hyperuricemia on the early detection of renal failure in a cohort of screened subjects. Hypertens Res. 2001;24:691-7.
- [Google Scholar]
- Plasma levels of high-energy compounds compared with severity of illness in critically ill patients in the intensive care unit. Surgery. 1998;124:65-72.
- [Google Scholar]
- Measures of total free radical activity in critically ill patients. Clin Biochem. 1999;32:263-8.
- [Google Scholar]
- Elevated uric acid increases the risk for acute kidney injury. Am J Med. 2012;125:302.e9-17.
- [Google Scholar]
- Serum uric acid as a predictor of mortality and future exacerbations of COPD. Eur Respir J. 2014;43:43-53.
- [Google Scholar]
- Increased urinary loss of uric acid in adults with acute respiratory failure requiring mechanical ventilation. CHEST Journal. 1992;1;102(2):556-9.
- [Google Scholar]
- Serum total antioxidant capacity reflects severity of illness in patients with severe sepsis. Crit Care. 2006;10:R36.
- [Google Scholar]





