Translate this page into:
Spectrum and Trends of Intestinal Parasitic Infections at a Tertiary Care Hospital during Pandemic Times: A Laboratory-Based Retrospective Study
Address for correspondence: Suneeta Meena, MD, Department of Laboratory Medicine, All India Institute of Medical Sciences, Delhi 110029, India (e-mail: suneetameena@gmail.com).
-
Received: ,
Accepted: ,
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction
Intestinal parasitic infections continue to loom in developing countries with low sanitation and socioeconomic conditions. Pandemic times are especially important to study the prevalence of these pathogens since the focus of all healthcare services was coronavirus disease 2019 (COVID-19). This study aimed to evaluate the prevalence and time-trend of intestinal parasitic infections in the capital region of India during the pandemic times.
Methods
In this cross-sectional study, a retrospective review based on data from the past 2 years in the post-COVID-19 pandemic was used. Descriptive and time-trend analyses were applied to the data. Time series analysis was analyzed using the best fit autoregressive integrated moving average (ARIMA) model to look for seasonality in trends and forecasting.
Results
A total of 7267 patients' stool samples over a 2-year pandemic period were included in the study. Intestinal parasites were detected in 11.18% (813/7276) patients. Giardia lamblia (2.28%) and Blastocystis hominis (3.78%) were the predominant ones. Time-trend analysis from 2020 to 2021 using ARIMA model predicted an increasing trend with waning of pandemic. The most prevalent infection was found in the monsoon and autumn months.
Conclusion
Rates of infection with Giardia lamblia and Blastocystis hominis have increased in comparison to other protozoan infections like Entamoeba histolytica when compared with prepandemic hospital-based studies. With fading of the pandemic, further increasing trends are predicted.
Keywords
prevalence
intestinal parasites
North India
pandemic
Introduction
Intestinal parasites are responsible for infecting approximately a quarter of world's population.[1] Prevalence in developing countries is even higher.[2] Several Indian studies have shown significant prevalence of intestinal parasitic infections in both hospital and community settings.[3,4] To this effect Government of India (Ministry of Health and Family Welfare) celebrates National Deworming Day on 10 February every year since 2015 for school-going children.[5] Implementation of this program has brought substantial reduction in prevalence of soil transmitted helminths.[6] But intestinal parasitic infections still continue to loom in the community and hospital settings.[7] So, regular assessments are essential to evaluate impact of the program. In this regard, coronavirus disease 2019 (COVID-19) pandemic that engulfed the whole world for last 2 years has posed a unique challenge, effect of which have been several. During lockdown both outpatient and elective services were restricted. In the department of laboratory medicine, stool samples are received for routine microscopic examination where all pathogenic findings are reported besides intestinal parasites. Hence, this study setting was appropriate to study prevalence of intestinal parasites where samples from both inpatients and outpatients are received for routine microscopic examination. This study aimed to assess the prevalence of intestinal parasitic infections among patients referred to the department of laboratory medicine for stool microscopic examination.
Methods
This retrospective study was conducted from January 2020 to December 2021 among patients referred to the department of laboratory medicine for stool microscopic examination. In this cross-sectional study, a retrospective review based on data of the past 2 years in post-COVID-19 pandemic was used. The study participants were inpatients and outpatients referred to All India Institute of Medical Sciences, Delhi. All participants gave stool samples for laboratory diagnosis during the study period. Then, the sociodemographic data of participants and laboratory results were collected from the available data of the hospital information system database.
For parasitological analysis, fresh stool samples were collected in the prelabeled wide-mouth plastic containers. Stool specimen was examined microscopically using the direct wet-mount.[8] Modified acid fast staining was done for samples of children and immunocompromised patients.[9]
Statistical Analysis
Descriptive statistics were presented as means or medians as appropriate for continuous variables and proportions for categorical variables. The comparison of normally distributed continuous variables between the groups was performed using Student's t-test. Nominal categorical data between the groups were compared using Pearson's chi-squared test. Non-normal distribution continuous variables were compared using Mann–Whitney U test. For all statistical tests, a p-value less than 0.05 will be taken to indicate a significant difference. The data were analyzed using SPSS Statistics for windows version 26, IBM Corp. Univariate time series data were compared with the monthly prevalence of intestinal parasites of each pathogen among total samples examined. Time series analysis was analyzed using the best-fit autoregressive integrated moving average (ARIMA) model to look for seasonality in trends and forecasting.
Results and Discussion
In the current study, datasets of 11,715 stool samples during the pandemic period (January 2020–December 2021) were obtained, extracted, tabulated, and assessed. Duplicates and consecutive samples were excluded. Finally, a total of 7,267 patients' stool samples over 2-year pandemic period were included in the study. The mean age of patients was 40.81 ± 20.74 standard deviation. Patients were divided into five major age groups of less than or equal to 14, 15 to 29, 30 to 44, 45 to 59, and more than or equal to 60. Although young adult patients were maximum in number, other groups were also present in equal preponderance. More than half of the patients were males (n = 4,125; 58%; Table 1).
Intestinal parasites were detected in 11.18% (813/7276) of patients. Blastocystis hominis was the predominant followed by Giardia lamblia, Entamoeba histolytica/E. dispar, and Cryptosporidium among others. Protozoan infections were clearly more common than helminthic infections. Endolimax nana, Entamoeba coli, Iodamoeba butschlii, and other protozoa with limited clinical implications were also seen in increased frequency (►Table 1). Modified acid-fast staining was done for samples of children and immunocompromised patients. Only Cryptosporidium spp. was detected in 10 (0.14%) patients. Soil transmitted helminths like Ascaris lumbricoides and Trichuris trichiura were occasionally detected. However, hookworm was detected in seven (0.1%) patients and Enterobius vermicularis was detected in four patients (0.06%). Mixed infections were detected in 157 (3.84%) patients. Single infections were predominant then mixed infections. Detection of Giardia lamblia and Blastocystis hominis singly and in combination with other intestinal pathogens is depicted in ►Fig. 1.
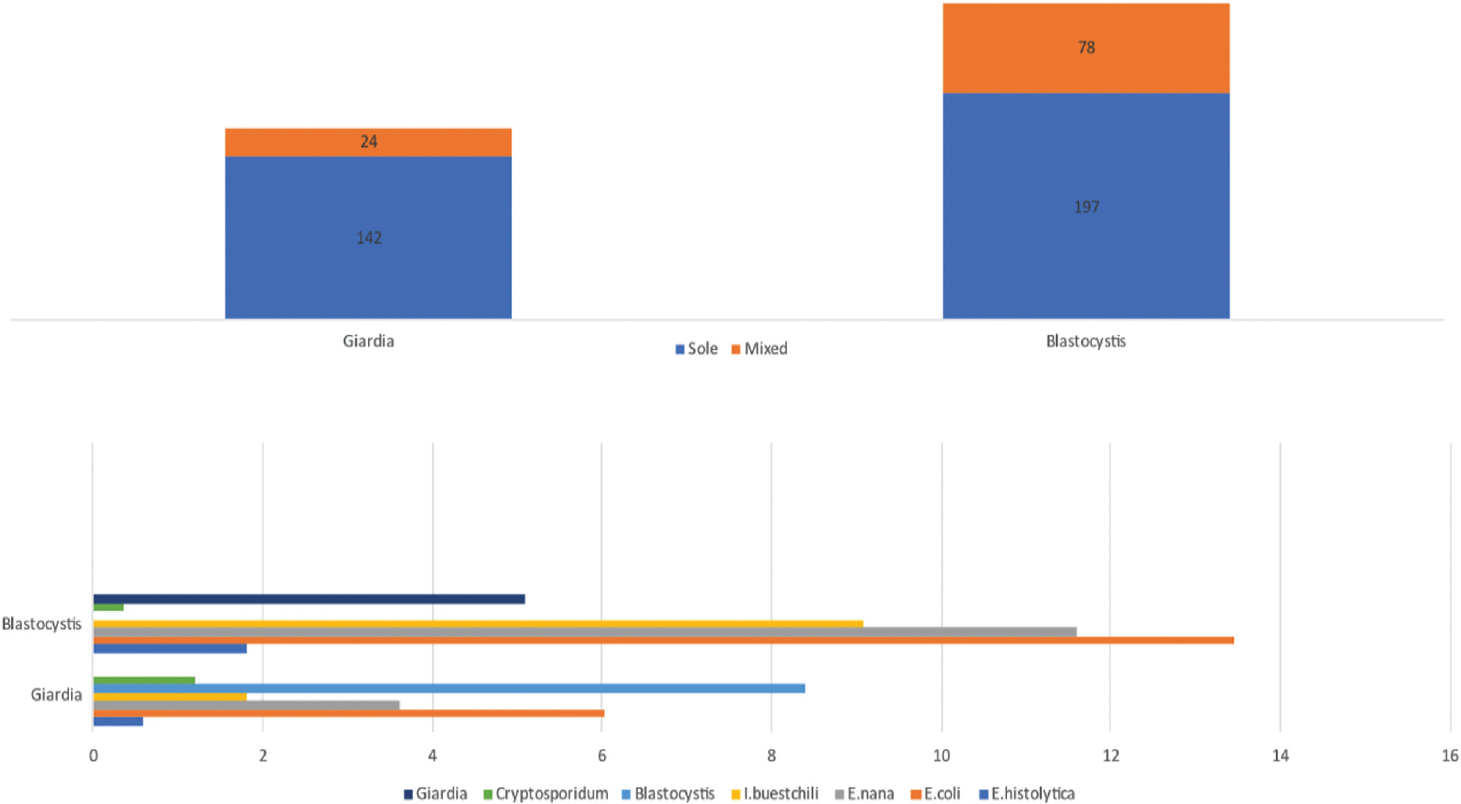
- Mixed infections of Giardia lamblia and Blastocystis hominis with other intestinal parasites.
| Parasite | Sex, n (%) | p-Value | Age, n (%) | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | ≤ 14 | 15–29 | 30–44 | 45–59 | ≥ 60 | |||
| 1. Ascaris | 1 (0.01) | 0 | 0.394 | 0 | 0 | 0 | 1 (0.04) | 0 | 0.388 |
| 2. Hookworm | 4 (0.05) | 3 (0.06) | 0.963 | 2 (0.2) | 1 (0.03) | 0 | 1 (0.04) | 3 (0.1) | 0.218 |
| 3. Taenia species | 1 (0.01) | 0 | 0.395 | 0 | 0 | 1 (0.03) | 0 | 0 | 0.515 |
| 4. Enterobius vermicularis | 2 (0.02) | 2 (0.04) | 0.746 | 0 | 1 (0.03) | 0 | 3 (0.13) | 0 | 0.072 |
| 5. Strongyloides stercoralis | 1 (0.01 | 0 | 0.394 | 1 (0.1) | 0 | 0 | 0 | 0 | 0.515 |
| 6. Giardia lamblia | 124 (1.8) | 42 (0.8) | <0.001 | 34 (3.5) | 45 (1.6) | 39 (1.4) | 27 (1.16) | 21 (0.7) | <0.001 |
| 7. E. histolytica/E. dispar | 8 (0.1) | 10 (0.2) | 0.243 | 1 (0.1) | 5 (0.18) | 2 (0.07) | 6 (0.25) | 4 (0.13) | 0.495 |
| 8. Cryptosporidium | 7 (0.1) | 3 (0.06)) | 0.442 | 2 (0.2) | 0 | 3 (0.1) | 2 (0.08) | 3 (0.1) | 0.421 |
| 9. Trichomonas | 0 | 1 (0.02) | 0.241 | 0 | 0 | 1 (0.03) | 0 | 0 | 0.515 |
| 10. Entamoeba coli | 148 (2.1) | 97 (1.98) | 0.438 | 16 (1.6) | 53 (1.9) | 68 (2.5) | 45 (1.9) | 63 (2.1) | 0.212 |
| 11. Entamoeba coli | 107 (1.56) | 80 (1.64) | 0.826 | 7 (0.7) | 56 (2.03) | 48 (1.7) | 28 (1.2) | 48 (1.6) | 0.014 |
| 12. Iodamoeba butschlii | 50 (0.7) | 37 (0.75) | 0.921 | 4 (0.4) | 28 (1.01) | 20 (0.7) | 16 (0.7) | 19 (0.63) | 0.293 |
| 13. Blastocystis hominis | 161 (2.3) | 114 (2.3) | 0.852 | 15 (1.5) | 88 (3.1) | 50 (1.8) | 43 (1.8) | 79 (2.6) | <0.001 |
| Total | 614 (61.2) | 389 (38.8) | 82 (8.1) | 277 (27.6) | 232 (23.1) | 172 (17.1) | 240 (23.9) | ||
| Prevalence | 614/6837 (8.9) | 389/4878 (7.9) | 82/951 (8.6) | 277/2756 (10) | 232/2702 (8.58) | 172/2314 (7.4) | 240/2988 (8.1) | ||
Across age groups, the highest prevalence of intestinal parasites was found in the age group of 16 to 30 (10%), whereas the lowest prevalence (7.1%) was in the age group 45 to 59 years. Giardia lamblia was the dominant parasite across all age groups. Highest prevalence of intestinal parasites was seen in children less than 14 years (4.1%). A decreasing trend was observed for Giardia lamblia and an increasing trend for Blastocystis species was observed with age. Age group wise trends and distribution in different age groups are depicted in ►Table 1.
Although male patients were more than half of the total patients, there was no significant difference in distribution of intestinal parasites in the two genders. However, a significant male preponderance was seen for Giardia lamblia.
Time-related trends of common intestinal parasites were analyzed using ARIMA model and a forecast was also predicted as shown. Seasonality was observed for Giardia lamblia even during pandemic times as is seen in both observed trend and predicted trend using ARIMA model. An increasing trend was seen for Blastocystis species (►Fig. 2). Mostly these infections were dominant in monsoon season and autumn period. A slight surge was also seen in winter months of year 2020 but the same surge was not reflected in 2021 as country was dealing with Omicron variant during that time (►Fig. 3). Further, with waning of pandemic increasing trends are predicted for common intestinal parasite.

- Autoregressive integrated moving average model for intestinal parasites versus the actual time series.

- Frequency of intestinal parasites during the study period month wise.
Prevalence of intestinal parasitic infections was 11.18% that is comparable to studies from Delhi and other parts of India. Prevalence is comparable to prepandemic studies (►Table 2).[3,10–12] In our study, other coccidian parasites like Cystoisospora and Cyclospora species were not seen. Increased prevalence of Cryptosporidium species as compared with other coccidian parasites has been reported in other studies as well.[3,13] Modified acid fast staining is done for samples of children and immunocompromised patients. Cryptosporidium species was detected in 10 (0.14%) patients. Moreover, stool concentration was not uniformly done for all samples that could be the reason for low prevalence of intestinal coccidian parasites.
| Current study | Uppal et al 2014[10] | Praharaj et al 2017[3] | Ghoshal et al 201618 | Singh et al 201619 | Mukherjee et al 2009[11] | |
|---|---|---|---|---|---|---|
| Study group | Hospital based | <12 years, symptomatic | Hospital based | Immunocompetent patients | Children <15 years | Hospital based |
| Study design | Cross-sectional retrospective | Cross-sectional prospective | Cross-sectional retrospective | Cross-sectional | Cross-sectional | Cross-sectional |
| Duration | Jan 2020–Dec 2021 (2 years) | 2012–2014 (2 years) | 2006–2012 (7 years) | 2008–2012 | 2015–2016 (6 months) | 2007–2008 (1 year) |
| Place | Delhi | Delhi | Vellore | Lucknow | Delhi | |
| Sample size | 7,267 | 6,527 | 257,588 | 80 | ||
| Overall | 11.18% | 4.78% | 8.9% | 20% | ||
| Giardia lamblia | 2.28% | 2.27% | 3.51% | 1.25% | 13.3% | |
| Blastocystis | 3.78% | 0.065 | 0.22% | |||
| Entamoeba histolytica/Entamoeba dispar | 0.25% | 0.64% | 0.82% | 0.72% | 4.6% | |
| Cryptosporidium | 0.14% | 0.15% | 0.73% | 10% | 5% | 7.6% |
| Cystoisospora | 0.13% | 4.9% | 2% | |||
| Cyclospora | 1.25% | 2.5% | ||||
| Hookworm | 0.06% | 0.03% | 1.6% | |||
| Ascaris | 0.01% | 1.15% | 1% | |||
| Hymenolepis nana | 0.25% | 3.3% | ||||
| Taenia | 0.04% | |||||
| Enterobius vermicularis | 0.06% | 0.01% | 2% | |||
| Strongyloides stercoralis | .01% | 0.1% | 0.9 | |||
| Trichuris trichiura | ||||||
| Balantidium coli | ||||||
| Mixed infections |
Prevalence of intestinal parasites was variable across different age groups but was highest in children (<14 years). Our results are in concordance with other studies.[3,14]
In our study, males (2.1%) had higher prevalence of intestinal parasites as compared with females (1.2%). A significant high male preponderance was found for Giardia. Increased prevalence in males can be influenced by environmental factors, eating habits, and hormonal difference between the sexes.[15] Sex hormones have previously been reported to play critical role in host defense and pathogen life cycle that could possibly explain our study results.[16]
Significant seasonality was seen for Giardia lamblia even during pandemic times. These trends have also been observed in other prepandemic studies from other settings.[3] Trends of intestinal parasites detected in the laboratory have also been influenced by different waves of pandemic as can be seen in results (►Fig. 3). Similar results have also been seen in another study where preventive measures imposed to tackle pandemic like, lockdown, have affected intestinal parasite prevalence.[17]
Limitations
Since it was primarily a laboratory-based study so clinical data could not be included. Modified acid-fast staining for intestinal parasites was done for sample of children and immunocompromised patients like people living with human immunodeficiency virus or transplant recipients. So, exact burden of coccidian parasites could not be determined. Moreover, stool concentration is not performed for all samples that could have influenced prevalence of intestinal coccidian parasites and helminths. Nonetheless, laboratory setup is an appropriate setting to study prevalence since it caters to both inpatients and outpatients.
Conclusion
Due to unavailability of recent prevalence study on intestinal parasites, this study had some interesting observations. Rates of infection with Giardia lamblia and Blastocystis hominis have increased in comparison to other protozoan infections like Entamoeba histolytica when compared with prepandemic hospital-based studies. However, their role in causing symptoms remains to be determined due to lack of clinical data. These findings have important public health implications.
Acknowledgment
Mrs Omvati Vats, Mr. Rajesh Gaurwal, Mr. Anand Kumar are involved in processing of stool samples. Riya Sayal and Manoj Kumar helped out in data compilation.
Conflict of Interest
None declared.
Funding
None.
References
- Prevalence and associated factors of intestinal parasitic infections among patients attending Shahura Health Center, Northwest Ethiopia. BMC Res Notes. 2019;12(01):333.
- [CrossRef] [PubMed] [Google Scholar]
- Intestinal amoebiasis: 160 years of its first detection and still remains as a health problem in developing countries. Int J Med Microbiol. 2020;310(01):151358.
- [CrossRef] [PubMed] [Google Scholar]
- Temporal trends of intestinal parasites in patients attending a tertiary care hospital in South India: a seven-year retrospective analysis. Indian J Med Res. 2017;146(01):111-120.
- [CrossRef] [PubMed] [Google Scholar]
- Intestinal parasitic infection in Bhil tribe of Rajasthan, India. J Parasit Dis. 2012;36(02):143-148.
- [CrossRef] [PubMed] [Google Scholar]
- Accessed March 15, 2023 at: https://www.nhp.gov.in/national-deworming-day_pg (accessed )
- Accessed March 15, 2023 at: https://pib.gov.in/pib.gov.in/Pressreleaseshare.aspx?PRID=1666053 (accessed )
- Prevalence and distribution of soil-transmitted helminth infections in India - PubMed [Internet] Accessed March 15, 2023 at: https://pubmed.ncbi.nlm.nih.gov/28209148/ (accessed )
- [Google Scholar]
- Practical guidance for clinical microbiology laboratories: laboratory diagnosis of parasites from the gastrointestinal tract. Clin Microbiol Rev. 2017;31(01):e00025-e17.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and microbiology profile and outcome of diarrhea by coccidian parasites in immunocompetent children. Pediatr Infect Dis J. 2015;34(09):937-939.
- [CrossRef] [PubMed] [Google Scholar]
- A comparative study of bacterial and parasitic intestinal infections in India. J Clin Diagn Res. 2015;9(03):DC01-DC04.
- [CrossRef] [PubMed] [Google Scholar]
- Hospital-based surveillance of enteric parasites in Kolkata. BMC Res Notes. 2009;2:110.
- [CrossRef] [PubMed] [Google Scholar]
- Coccidian intestinal parasites among immunocompetent children presenting with diarrhea: Are we missing them? Trop Parasitol. 2017;7(01):37-40.
- [Google Scholar]
- Intestinal coccidian parasites as an underestimated cause of travellers' diarrhoea in Polish immunocompetent patients. Acta Parasitol. 2017;62(03):630-638.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of intestinal parasites in a university hospital in Northern Cyprus: a 4-year retrospective experience. Turkiye Parazitol Derg. 2021;45(02):128-132.
- [CrossRef] [PubMed] [Google Scholar]
- Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunol. 2004;26(6-7):247-264.
- [CrossRef] [PubMed] [Google Scholar]
- Sexual dimorphism and gender in infectious diseases. Front Immunol. 2021;12 698121 https://www.frontiersin.org/articles/10.3389/fimmu.2021.698121 [Internet] (accessed )
- [CrossRef] [PubMed] [Google Scholar]
- The impact of COVID-19 pandemic on access to healthcare: the experience of the Diagnostic Parasitology Laboratory of Ege University. Turkiye Parazitol Derg. 2022;46(02):124-128.
- [CrossRef] [PubMed] [Google Scholar]






