Translate this page into:
Study of Hemato-morphological Features in Neuroblastoma Infiltrating Marrow
Address for correspondence: Rashmi Kushwaha, MD, Department of Pathology, King George Medical University, Lucknow, 226001, Uttar Pradesh, India (e-mail: rashmikushwaha@kgmcindia.edu).
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective
Neuroblastoma typically affects children within the first 5 years of life and accounts for 10% of all pediatric malignancies. Neuroblastoma at onset may manifest as a localized or metastatic illness. The aim of this study was to identify hematomorphological features in neuroblastoma infiltrating marrow as well as to ascertain the prevalence of bone marrow infiltration in neuroblastoma.
Materials and Methods
This retrospective study included newly diagnosed 79 cases of neuroblastoma, which were referred for bone marrow examination for the staging of the disease. Medical records were retrieved to acquire hematomorphological findings of peripheral blood and bone marrow smears. Statistical Package for Social Sciences, IBM Inc., USA, version 21.0 was used to analyze the data.
Results
The interquartile age range of neuroblastoma cases was 24.0 to 72.0 months (median = 48 months) with a male to female ratio of 2.7:1. Also, 55.6% (44/79) of cases in the study population showed evidence of marrow infiltration. The bone marrow infiltration was significantly linked to thrombocytopenia (p = 0.043) and nucleated red blood cells (p = 0.003) in peripheral blood. The bone marrow smears of cases with infiltration showed a significant shift to the left in the myeloid series (p = 0.001) and an increased number of erythroid cells (p = 0.001).
Conclusion
For neuroblastoma patients, a diligent, exhaustive search for infiltrating cells in bone marrow is advised if thrombocytopenia or nucleated red blood cells are identified on a peripheral blood smear and bone marrow smears showed myeloid left shift with an increased number of erythroid cells.
Keywords
neuroblastoma
bone marrow
peripheral blood
thrombocytopenia
nucleated red blood cells
Introduction
A pediatric tumor termed neuroblastoma (NB) develops from the peripheral sympathetic nervous system. Neuroblastoma is specifically produced from neural crest (NC)-derived cells that are undergoing defective sympathetic neuronal evolution due to genomic and epigenetic abnormalities (NB). Although it can occur everywhere that migrating neural crest cells (NCCs) and their progeny can reach, NB most usually occurs in the paraspinal ganglia or the adrenal medulla (AM).[1] The most prevalent solid extracranial tumor in children, accounting for 10% of pediatric malignancies and 50% of those in infants, is neuroblastoma. It is a pediatric tumor, 36% of children who are diagnosed with it are under 1 year old, 75% are under 5, and more than 90% are under 10 years old.[2–4] Patients are divided into risk categories according to many prognostic criteria, such as age at diagnosis, stage, histological characteristics, and molecular abnormalities. NB at onset may manifest as a localized or metastatic illness. NB is a major cause of death from childhood cancer, especially in high-risk children with chemo-resistant recurrence, whose survival rate is only approximately 40%.[5]
Another justification for precisely determining the state of the marrow in newly diagnosed instances is autologous bone marrow “rescue.”[6] The International Neuroblastoma Staging System (INSS) classifies a pediatric tumor that arises from the developing peripheral sympathetic nervous system. Involvement of the bone marrow, which is classified as stage 4, is common in patients with advanced disease. For the purpose of evaluating the marrow infiltration in solid tumors, several studies have been performed.[7–9] Only a few studies have explored the marrow infiltration in neuroblastoma and its relationship to hematological parameters.[10–12]
Aims and Objectives
The aim of this study was to identify hematomorphological features in neuroblastoma infiltrating marrow as well as to ascertain the prevalence of bone marrow infiltration in neuroblastoma.
Materials and Methods
This study was conducted in the Department of Pathology, King George Medical University, Lucknow, a tertiary care center in the state of Uttar Pradesh, North India. The study included newly diagnosed cases of neuroblastoma presented for a bone marrow examination between January 2018 to May 2022 for the staging of the disease. The clinico-radiological characteristics, histopathology, and immunohistochemistry findings were used to make the primary diagnosis of neuroblastoma in all cases. The posterior superior iliac spine was used for the bone marrow procedure. After that, according to established procedures, aspirate smears and biopsy sections were stained with May-Grunwald Giemsa and hematoxylin and eosin stain, respectively.[13,14] The complete blood count, peripheral blood smear examination, bone marrow morphological features, as well as the presence or absence of neuroblastoma infiltration and immunohistochemistry findings were retrieved from the records of each patient's bone marrow case sheets.
Statical Analysis
The Statistical Package for Social Sciences (SPSS, IBM Inc., USA) version 21.0 was used to analyze the data. To compare categorical data, the chi-square test was utilized, while independent t-tests were used to analyze parametric data. A statistically significant value was defined as one with a p-value less than 0.05.
Results
All neuroblastoma patient records that had a bone marrow examination between January 2018 and May 2022 were retrieved from bone marrow case sheets. Records of total 103 cases of neuroblastoma were retrieved. The cases that received chemotherapy (24/103) and a bone marrow examination conducted to check for any residual disease were excluded from the study because there was no information of their bone marrow condition at the time of diagnosis. The final study included 79 newly diagnosed patients with neuroblastoma for the staging of the disease (►Fig. 1).
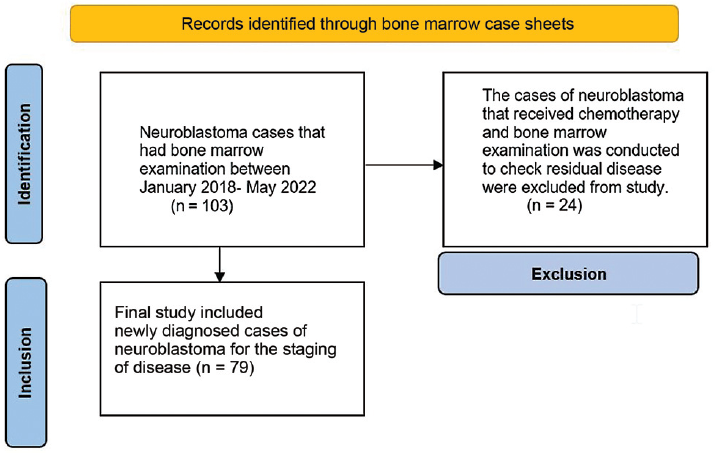
- PRISMA flow chart of the identification and inclusion of neuroblastoma cases.
The interquartile age range of neuroblastoma cases was 24.0 to 72.0 months (median = 48 months) with a male to female ratio of 2.7:1. Two bone marrow aspirates, two trephine biopsy touch imprints, a bone marrow biopsy, and a battery of immunohistochemistry marker were evaluated in each case. All findings were retrieved from the bone marrow case sheet records. Marrow infiltration was noted in 55.6% (44/79) of the neuroblastoma cases (►Table 1).
| Parameters | Total cases (n = 79) | Cases with marrow infiltration (n = 44) | Cases without marrow infiltration (n = 35) | p-Value | |
|---|---|---|---|---|---|
| Age (mo) | Mean ± SD | 50.91 ± 34.52 | 50.02 ± 34.48 | 52.03 ± 35.05 | 0.799 |
| Median | 48.00 | 48.00 | 48.00 | ||
| Interquartile Range | 24.0–72.0 | 24.0–72 | 24.0–72 | ||
| Gender (n, %) | Male | 58 (73.42%) | 32 (72.73%) | 26 (74.29%) | 0.876 |
| Female | 21 (26.58%) | 12 (27.27%) | 9 (25.71%) | ||
| Hemoglobin (Hb) | Mean ± SD | 9.04 ± 2.33 | 8.67 ± 2.41 | 9.51 ± 2.17 | 0.115 |
| Median | 9.10 | 8.80 | 9.80 | ||
| Interquartile range | 7.20–10.70 | 6.93–9.80 | 7.30–11.20 | ||
| Total leucocyte count (× 109/L) | Mean ± SD | 10.61 ± 4.05 | 10.01 ± 3.65 | 11.37 ± 4.43 | 0.137 |
| Median | 10.00 | 10.00 | 11.00 | ||
| Interquartile range | 8.50–12.90 | 8.08–12.78 | 8.90–13.00 | ||
| Platelet count (× 109/L) | Mean ± SD | 305 ± 181 | 258 ± 186 | 363 ± 158 | 0.009* |
| Median | 300 | 240 | 350 | ||
| Interquartile range | 180–450 | 86–388 | 270–450 | ||
*Significant (p < 0.05).
The complete blood count of neuroblastoma cases with marrow infiltration revealed anemia in 81.2% (36/44) of the cases with an interquartile range of hemoglobin (Hb) 6.93 to 9.80 g/dL (median = 8.80 g/dL), leukopenia in 9.09% (4/44) of cases with an interquartile range of total leucocyte count (TLC) 8.08 × 109/L to 12.7 × 109/L (median = 10.0 × 109/L) and thrombocytopenia in 29.55% (13/44) of cases with an interquartile range of platelet count (PC) 86 × 109/L to 388 × 109/L (median = 240 × 109/L) (►Table 1).
It was observed that bone marrow involvement in cases of neuroblastoma was significantly linked to the presence of thrombocytopenia (p = 0.043) and nucleated red blood cells (nRBCs) (p = 0.003) in peripheral blood. All remaining hematological parameters such as anemia (p = 0.594), leukopenia (p = 0.189), and bicytopenia (p = 0.369) were not significantly correlated with marrow infiltration in neuroblastoma cases. Pancytopenia was present in 9.09% (4/44) of cases of neuroblastoma with marrow infiltration; however, none of the cases of neuroblastoma without marrow infiltration showed pancytopenia (p = 0.189) (►Table 2).
| Parameters | Total cases (n = 79) | Cases with marrow infiltration (n = 44) | Cases without marrow infiltration (n = 35) | p-Value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Anemia | 62 | 78.48 | 36 | 81.82 | 26 | 74.29 | 0.594 |
| Leucopenia | 4 | 5.06 | 4 | 9.09 | 0 | 0.00 | 0.189 |
| Thrombocytopenia | 16 | 20.25 | 13 | 29.55 | 3 | 8.57 | 0.043* |
| Bicytopenia | 11 | 13.92 | 8 | 18.18 | 3 | 8.57 | 0.369 |
| Pancytopenia | 4 | 5.06 | 4 | 9.09 | 0 | 0.00 | 0.189 |
| Nucleated red blood cells | 18 | 22.78 | 16 | 36.36 | 2 | 5.71 | 0.003* |
*Significant (p < 0.05).
The bone marrow aspirate smears findings were retrieved from records in each case. The bone marrow smears showed a significant left shift in myeloid series (p = 0.001) and an increased percentage of erythroid cells (p = 0.001) in cases with bone marrow infiltration by neuroblastoma in comparison to cases without bone marrow involvement. The percentage of lymphocytes (p = 0.497) and plasma cells (p = 0.646) were not significantly linked with cases of neuroblastoma infiltrating marrow (►Table 3).
| Percentage of bone marrow nucleated cells | Total cases (n = 79) | Cases with marrow infiltration (n = 44) | Cases without marrow infiltration (n = 35) | p-Value | |||
|---|---|---|---|---|---|---|---|
| Mean | ± SD | Mean | ± SD | Mean | ± SD | ||
| Promyelocyte | 1.61 | 0.84 | 1.84 | 0.96 | 1.31 | 0.53 | 0.005* |
| Myelocyte | 18.81 | 7.27 | 22.11 | 7.22 | 14.66 | 4.85 | < 0.001* |
| Metamyelocyte | 12.87 | 5.75 | 15.91 | 5.63 | 9.06 | 2.97 | < 0.001* |
| Band forms + segmented neutrophils | 20.25 | 13.46 | 10.57 | 6.63 | 32.43 | 9.28 | < 0.001* |
| Erythroid cells | 33.70 | 9.91 | 37.27 | 10.52 | 29.20 | 6.94 | < 0.001* |
| Lymphocytes | 11.90 | 6.14 | 11.48 | 6.78 | 12.43 | 5.27 | 0.497 |
| Plasma cells | 2.04 | 1.22 | 2.12 | 1.17 | 1.95 | 1.29 | 0.646 |
*Significant (p < 0.05).
Trephine biopsy findings were also retrieved from records in each case. In 36 of the 44 positive cases, trephine biopsy and aspirate smear both revealed infiltration. In five cases, only aspirate smears and in another three cases, only trephine biopsies were positive. In five cases where infiltration was detected only by aspirate, biopsy sections primarily revealed diffuse fibrosis, and in cases where infiltration was detected only by biopsy, there was localized involvement that was missed by aspiration due to improper sampling. The most prevalent pattern of infiltration was rosette formation, which was observed in 31.8% (14/44) of cases on trephine biopsies and in 52.2% (23/44) of cases on aspirate smears (►Fig. 2A, B) (►Fig. 2C, D). The remaining cases displayed an interstitial focal, and diffuse pattern of infiltration in bone marrow biopsies. Immunohistochemistry was applied in all cases and found positive for synaptophysin and chromogranin (►Fig. 2E, F). In atypical cells infiltrating the bone marrow, other immunohistochemistry markers of round cell malignancy such as leucocyte common antigen (LCA), CD99, desmin, and myogenin were not expressed.

- Bone marrow aspirate, bone marrow biopsy and immunohistochemistry findings of bone marrow examination. (A) Bone marrow aspirate smear showing infiltration by neuroblastoma (May-Grunwald-Giemsa,100×) (B) Bone marrow aspirate smear showing infiltration by neuroblastoma with numerous rosettes (May-Grunwald-Giemsa, 400×) (C) Hematoxylin and eosin stained histologic section of bone marrow trephine biopsy showing infiltration by neuroblastoma (H&E, 100×) (D) Hematoxylin and eosin stained histologic sections showing bone marrow trephine biopsy displaying neuroblastoma infiltration with numerous rosettes (H&E, 200×) (E) Bone marrow biopsy displaying positive expression of chromogranin antibody in tumor cells (200×) (F) Bone marrow biopsy displaying positive expression of synaptophysin antibody in tumor cells (200×).
Discussion
Neuroblastoma is an embryonic tumor that develops from cells in the neural crest, an unstable structure made up of multipotent stem cells active in the early stages of embryonic development that arise along the borders of the closing neural tube.[15] An estimated two-thirds of primary tumors are found in the abdomen. It has the potential to spread to the local lymph nodes as well as distant locations, primarily the bone marrow, cortical bone, liver, and skin. Paraneoplastic disorders such as OMAS (opsoclonus-myoclonus-ataxia syndrome) or persistent watery diarrhea can also be symptoms of neuroblastoma.[16] All neuroblastoma patients must have their marrow examination for staging purposes; if invaded, the patient falls into a high-risk category and needs intensive chemotherapy.[7] A poor prognosis is nearly invariably associated with bone marrow metastases. The homing of tumor cells to the marrow and the ensuing bone metastases are largely the result of interactions between the tumor cells and the BM microenvironment.[17] In this study, 44 patients (55.6%) showed bone marrow infiltration by neuroblastoma. Pulkit et al[18] found similar findings and noted marrow involvement in 54.5% of cases under the age of 5 years, with male predominance. Cozzutto et al[19] also observed similar findings in 58.3% of cases (7 out of 12 cases), and Franklin et al[20] reported a similar frequency in 48.9% of cases (24 out of 49 cases). According to Madhumati et al's study, neuroblastoma is a non-hematological tumor that affects the bone marrow most frequently (48.8%), followed by retinoblastoma (11.1%), Ewing's sarcoma/primitive neuroectodermal tumor (PNET) (8.6%), and rhabdomyosarcoma (3.2%).[21] In our study, thrombocytopenia and the presence of nucleated red blood cells (nRBCs) in patients with and without marrow invasion showed a statistically significant difference. Bone marrow involvement causes inhibition of normal hematopoiesis, which results in peripheral cytopenias. This study showed pancytopenia in 9.09% (4/44) of the cases with marrow infiltration; however, not a single case of neuroblastoma without marrow infiltration showed pancytopenia (p = 0.189). No significant correlation was noted for anemia in patients of neuroblastoma with and without infiltration, presumably due to the frequency of iron insufficiency in India rather than related to the illness itself. Similar findings have also been described in prior studies.[7,8] In cases of neuroblastoma with bone marrow infiltration, the aspirate smears from the bone marrow demonstrated a significant left shift in myeloid series (p = 0.001) and an increased percentage of erythroid cells (p = 0.001) when compared with cases without bone marrow infiltration. We could not compare our results due to the unavailability of such literature even after an extensive search. Patients who have metastases may benefit from therapeutic intensification because they have advanced disease stages, which indicate a poor clinical prognosis. It is advised that these individuals get routine bone marrow screening for staging and treatment. Bone marrow examination, however, is an easy, uncomplicated, and affordable method used to stage and monitor these patients[22] and is ideal in resource-constrained situations in third-world nations. The weakness of this study was not employing molecular investigations, which can help in detecting minor marrow infiltration.
Conclusion
For neuroblastoma patients, a diligent, exhaustive search for infiltrating cells in bone marrow is advised if thrombocytopenia or nucleated red blood cells are identified on a peripheral blood smear and bone marrow smears showed myeloid left shift with an increased number of erythroid cells. Bone marrow infiltration is a strong predictive feature of poor prognosis and is associated with a more advanced disease stage; hence, early diagnosis is essential. Staging and monitoring of patients in a setup with limited resources is simple and economical and is attributable to bone marrow examination.
Authors' Contributors
R.K. did study designing, conceptual analysis, data acquisition, and literature search. A.S. did literature search, and conceptual analysis and proof correction. S.R. did data analysis and designing. G.Y. did data analysis and proofreading. S.P.V. contributed to clinical studies and literature searches. U.S. Singh did study design and literature search.
Conflict of Interest
None declared.
Funding
None.
References
- Recent advances in the developmental origin of neuroblastoma: an overview. J Exp Clin Cancer Res. 2022;41(01):92.
- [CrossRef] [PubMed] [Google Scholar]
- Congenital malignant disorders. In: Ballard R, Avery M, Taeusch W, eds. Diseases of the newborn. Philadelphia: W.B.: Saunders Company; 1991. p. :1025-48.
- [Google Scholar]
- Neuroblastoma. In: Pochedly C. ed. Neoplastic diseases in childhood. Harwood Academic Publishers; 1994:735-78.
- [Google Scholar]
- Neuroblastoma. In: Pizzo PA, Poplack DG, eds. Principles and practice of pediatric oncology (2nd). Philadelphia: J.B. Lippincott Company; 1993. p. :739-68.
- [Google Scholar]
- Neuroblastoma: clinical and biological approach to risk stratification and treatment. Cell Tissue Res. 2018;372(02):195-209.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis of bone marrow metastases in children with solid tumors and lymphomas. Aspiration, or unilateral or bilateral biopsy? Arch Med Res. 2000;31(01):58-61.
- [CrossRef] [PubMed] [Google Scholar]
- Metastasis of solid tumors in bone marrow: a study from northern India. Indian J Hematol Blood Transfus. 2011;27(02):93-95.
- [CrossRef] [PubMed] [Google Scholar]
- Bone marrow metastasis in solid tumors. Indian J Pathol Microbiol. 2003;46(04):613-616.
- [Google Scholar]
- Metastasis of solid tumours in bone marrow: a study from Kashmir, India. J Clin Pathol. 2003;56(10):803.
- [CrossRef] [PubMed] [Google Scholar]
- Detection of neuroblastoma in the bone marrow: biopsy versus aspiration. J Pediatr Hematol Oncol. 1998;20(04):330-334.
- [CrossRef] [PubMed] [Google Scholar]
- Bone marrow changes in neuroblastoma. Pediatr Pathol. 1986;5(02):225-234.
- [CrossRef] [PubMed] [Google Scholar]
- Cyto-morphologic evaluation of bone marrow in infants with disseminated neuroblastoma. J Pediatr Hematol Oncol. 2012;34(02):154-158.
- [CrossRef] [PubMed] [Google Scholar]
- Bone Marrow Biopsy. In: Lewis SM, Bain BJ, Bates I, eds. Dacie and Lewis practical haematology (10th). Philadelphia: Churchill Livingstone; 2006. p. :115-130.
- [CrossRef] [PubMed] [Google Scholar]
- Preparation and staining methods for blood and bone marrow films. In: Lewis SM, Bain BJ, Bates I, eds. Dacie and Lewis practical haematology (10th). Philadelphia: Churchill Livingstone; 2006. p. :59-78.
- [CrossRef] [Google Scholar]
- Setting appropriate boundaries: fate, patterning and competence at the neural plate border. Dev Biol. 2014;389(01):2-12.
- [CrossRef] [PubMed] [Google Scholar]
- Neuroblastoma in a developing country: miles to go. Indian J Pediatr. 2019;86(05):403-405.
- [CrossRef] [PubMed] [Google Scholar]
- Homing of cancer cells to the bone. Cancer Microenviron. 2011;4(03):221-235.
- [CrossRef] [PubMed] [Google Scholar]
- Bone marrow involvement in neuroblastoma: a study of hemato-morphological features. Indian J Hematol Blood Transfus. 2015;31(01):57-60.
- [CrossRef] [PubMed] [Google Scholar]
- Bone marrow biopsy in children: a study of 111 patients. Med Pediatr Oncol. 1979;6(01):57-64.
- [CrossRef] [PubMed] [Google Scholar]
- Detection of bone marrow invasion by neuroblastoma is improved by sampling at two sites with both aspirates and trephine biopsies. J Clin Pathol. 1983;36(11):1215-1218.
- [CrossRef] [PubMed] [Google Scholar]
- Bone marrow involvement at presentation in pediatric non-haematological small round cell tumours. Indian J Pathol Microbiol. 2007;50(04):886-889.
- [Google Scholar]
- Comparison of two methods for evaluating bone marrow metastasis of neuroblastoma: reverse transcription-polymerase chain reaction for tyrosine hydroxylase and magnetic resonance imaging. Pediatr Int. 2004;46(04):387-393.
- [CrossRef] [PubMed] [Google Scholar]






