Translate this page into:
Study of Invasive Pneumococcal Infection in Adults with Reference to Penicillin Resistance
Address for correspondence: Dr. Vrishali Avinash Muley, E-mail: vamuley@rediffmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Invasive pneumococcal infections often prove rapidly fatal, even where good medical treatment is readily available. In developed countries, up to 20% of people who contract pneumococcal meningitis die; however, in developing world, mortality is closer to 50%, even among hospitalized patients. The World Health Organization estimated 600,000–800,000 adult deaths each year from pneumococcal pneumonia, meningitis, and sepsis.
Aims:
This study aims to estimate isolation rate of invasive pneumococcal infection in adults, to determine the antimicrobial susceptibility profile of Streptococcus pneumoniae isolates and to study the associated risk factors.
Materials and Methods:
A total of 120 patients with suspected invasive infection such as meningitis, septicemia, and pleural effusion, were included in the study. Various clinical specimens such as pus, cerebrospinal fluid, and other sterile body fluids were processed for isolation and identification of S. pneumoniae. Kirby–Bauer disc diffusion method was performed to determine the antimicrobial susceptibility profile. Minimum inhibitory concentration test was performed to determine the penicillin resistance.
Results:
Of 120 patients, 40 (33.33%) cases were proven by culture to have an invasive pneumococcal infection. The most common clinical condition observed was meningitis followed by pneumonia with pleural effusion and sepsis. Pneumococcal isolates exhibited 40% resistance to cotrimoxazole and 12.73% to chloramphenicol. Two meningeal isolates exhibited penicillin resistance. Comorbidities observed in 21 (52.5%) cases were mainly Diabetes mellitus, smoking, and alcoholism.
Conclusions:
Invasive pneumococcal infection has poor prognosis and penicillin-resistant strains have become increasingly common. This study emphasizes the importance of judicious use of antibiotics, especially to refrain their use in mild self-limiting upper respiratory infections.
Keywords
Adults
invasive pneumococcal infection
penicillin resistance
INTRODUCTION
Spectrum of Streptococcus pneumoniae infection ranges from asymptomatic colonization of nasopharynx to mucosal disease to invasive infections.[1]
Invasive pneumococcal disease (IPD) is more common in children younger than 2 years, adults older than 65 years of age and those with underlying comorbidity or risk factor[23] with case fatality ranging from 26% to 30% in Asia and 11–30% in adults of the Western world.[456]
The vast majority of disease burden data and epidemiological studies on pneumococcal disease have been reported in child populations; however, limited data are available regarding the burden and epidemiology of pneumococcal disease in adults, particularly in Asia. Importantly, the population of Asia is the fastest growing population in the world, and identifying key strategies for combating pneumococcal disease has the greatest potential public health impact worldwide.[3]
The incidence of IPD is expected to rise secondary to the increase in the elderly populations. Groups most at risk of pneumococcal infection include those of extreme age, immunocompetent persons with underlying medical conditions (chronic cardiovascular, pulmonary, liver and neurological diseases, and diabetes mellitus), and individuals with defects of immune defenses or decreased immune responses (functional or anatomic asplenia, immunosuppressive conditions, postorgan or bone marrow transplantation, or on therapy with alkylating agents, antimetabolites, or systemic corticosteroids).
Other risk factors include male sex, alcohol abuse, cigarette smoking, asthma, cerebral spinal fluid leakage, cochlear implant, recent influenza infection, institutionalization, and certain ethnic groups.[7]
The isolation rate of antibiotic-resistant pneumococci has been increasing globally including India. The multicentric Asian Network for Surveillance of Resistant Pathogens Study (ANSORP) in Asia has documented distinctive increase in the penicillin and other antimicrobial resistance among S. pneumoniae isolates in Asian countries compared to Western world.[389]
A multidrug-resistant clone of pneumococcal serotype 19A (ST320 clone), has recently shown a high isolation rate in Asian countries and has also been isolated from India.[10]
The present study was undertaken to study the invasive pneumococcal infection in adults and to determine antimicrobial susceptibility profile of S. pneumoniae isolates.
MATERIALS AND METHODS
This study was a prospective observational study over a period of 1 year conducted in Department of Microbiology of a Tertiary Care Hospital.
Adult patients with suspected invasive infection admitted to the hospital were included in the study. Invasive infection included clinical conditions such as sepsis, meningitis, or pneumonia with pleural effusion. Clinical data regarding the age, time of disease onset, duration of fever, underlying diseases were recorded. Laboratory parameters such as hemoglobin, total white blood cell and differential count, and erythrocyte sedimentation rate were also noted. The various specimens received in the microbiology laboratory from these patients were processed by standard microbiological techniques. The various samples processed included pus, blood, cerebrospinal fluid (CSF), pleural fluid, and other sterile body fluid. S. pneumoniae was isolated by culture on 5% sheep blood agar and identified by sensitivity to optochin, bile solubility test, and other biochemical tests.[11] Antimicrobial susceptibility testing was done by Kirby–Bauer's disc diffusion method according to Clinical and Laboratory Standards Institute (CLSI) guidelines.[12] The antimicrobials tested by disc diffusion method were oxacillin 1 µg, erythromycin 15 µg, ciprofloxacin 5 µg, Chloramphenicol 30 µg, cotrimoxazole 25 µg, and cefotaxime 30 µg.
Oxacillin disc test with zone diameter ≤19 mm was used as an indicator of penicillin resistance. Resistance to penicillin was confirmed by minimum inhibitory concentration (MIC) method by broth dilution method using cation-adjusted Mueller-Hinton broth supplemented with 5% lysed horse blood. Penicillin resistance was interpreted using two separate criteria for meningeal and nonmeningeal isolates as per CLSI guidelines.[12]
Antimicrobial discs used were obtained from HiMedia. S. pneumoniae ATCC 49619 was used as a quality control strain.
Demographic data were obtained from clinical records.
RESULTS
A total of 120 patients with suspected invasive infection like sepsis, meningitis, or pneumonia with pleural effusion were included in the study. Of these, eighty cases (66.6%) were proven by culture to have an invasive bacterial infection. Of these culture positive eighty cases, 34 (42.5%) cases were of pneumonia with pleural effusion, 25 (31.25%) were of meningitis, and 21 (26.25%) cases were of sepsis. From these 80 cases, a total of 101 bacterial agents were isolated. Among these isolates, predominant isolates were of S. pneumoniae (55 isolates from 40 cases). Other isolates were Klebsiella pneumonaie (21 isolates from 18 cases), Staphylococcus aureus (14 isolates from 12 cases), Acinetobacter species (nine isolates from eight cases) and Salmonella typhi (two isolates from two cases).
Thus, in the present study, forty cases were proven by culture to have invasive pneumococcal infection documenting an isolation rate of 33.3%. Of these bacteriologically proven forty cases of invasive pneumococcal infection; 18 (45%) were of meningitis, 15 (37.5%) pneumonia with pleural effusion and 7 (17.5%) of sepsis. In seven cases of meningitis, S. pneumoniae were isolated from blood in addition to CSF. In pneumonia with pleural effusion, S. pneumoniae was isolated from sputum and blood from four cases each. Thus, from forty cases total 55 isolates of S. pneumoniae were obtained [Table 1].

Only two meningeal isolates showed resistance to penicillin with oxacillin zone diameter <19 mm. Penicillin MIC of these isolates were 0.25 mcg/ml and 0.50 mcg/ml respectively accounting to 3.64% penicillin resistance.
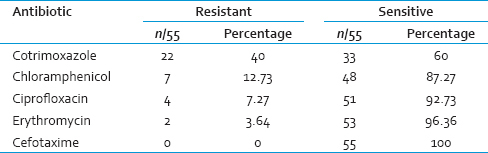
On further to antimicrobial susceptibility testing, 40% (22) isolates were resistant to cotrimoxazole followed by 12.73% (7) to chloramphenicol, 7.27% (4) to ciprofloxacin, and 3.64% (2) to erythromycin. All isolates were sensitive to cefotaxime [Table 2].
No isolate exhibited resistance to more than two antimicrobials tested.
Comorbidities observed in 21 (52.5%) cases were mainly diabetes mellitus, smoking, and alcoholism.
DISCUSSION
IPD shows differing population demographics. In this study, 33.3% cases were proven by culture to have an invasive pneumococcal infection. The invasive bacterial infections surveillance (IBIS) study has reported 43.3% cases of invasive pneumococcal infection[13] and study by Thomas et al. at Vellore has documented 53.9% cases of IPD.[6]
The most common clinical presentation was meningitis followed by pneumonia with pleural effusion. S. pneumoniae is known to be the most common cause of bacteremic meningitis in adults.[1]
In a 10 years retrospective study on community-acquired acute bacterial meningitis in India, S. pneumoniae accounted for >60% of cases in adults.[14]
The first case of penicillin-resistant pneumococcus was reported in 1967 from Australia. Subsequently, resistant strains began to appear in other parts of the world. In 1977, South African strains resistant to 2–10 µg/ml penicillin were reported.[15] Until the 1990s, S. pneumoniae was uniformly susceptible to all relevant antimicrobial agents.
ANSORP study in 11 Asian countries documented very high rates of penicillin and erythromycin resistance in S. pneumoniae as well as the introduction and spread of the 19A and Spanish 23F drug-resistant clones in Asia.[8]
In this study, complete resistance to penicillin was seen in two meningeal isolates accounting to 3.64% penicillin resistance. It is worth noting that, the multicentric IBIS study in Indian population revealed rising trend of penicillin resistance from 1.3% in 1993–1997 (Phase 1) to 7.4% in 1993–2003.[13] The study by Molander et al. from Vellore observed 4.5% penicillin resistance.[16]
Penicillin-resistant isolates display reduced affinity of penicillin-binding proteins (PBPs). The resistance is acquired by transformation and transposons and is not a phenomenon arising during treatment. The major reason for this is increased globalization causing geographic spread of drug-resistant clones with special capacity to spread and colonize facilitated by antibiotic pressure.[1]
Pneumococcal resistance to penicillin is fairly low on the Indian subcontinent but appears to be increasing, and this requires continued surveillance to observe the change in trend over a period.[13]
Furthermore, penicillin-resistant strains with altered PBPs are associated with concurrent resistance to third generation cephalosporins, macrolides, cotrimoxazole, tetracycline, and quinolones.[1]
The well-known multicentric study in India, IBIS, has reported increasing secular trend of penicillin, cotrimoxazole and cefotaxime resistant S. pneumoniae in India.[13]
In antimicrobial susceptibility pattern of isolates in this study, maximum resistance was observed for cotrimoxazole followed by chloramphenicol and ciprofloxacin. The Phase I and II of IBIS study showed increasing resistance to cotrimoxazole (56.3–75.9%) and a decrease in the rate of erythromycin and chloramphenicol resistance (erythromycin: 4.2–2.3% and chloramphenicol 16.6–13.4%). A study by Molander et al. stated 0.4% resistance to cefotaxime, a third generation cephalosporin.[16] No resistance was observed to cefotaxime in present study.
Symptomatology of pneumococcal infection is similar to many other infections. Most of the times, treatment is started without knowing the etiological agent and continued without any microbiological studies. Drugs such as amoxicillin, cotrimoxazole, and erythromycin are available easily over the counter and widely used for treatment of upper respiratory tract infection. Furthermore, drug-resistant S. pneumoniae is known to persist in the community in the form of asymptomatic nasopharyngeal carriage which forms part of the spectrum of pneumococcal infection.
Invasive pneumococcal infections and spread of drug-resistant strains can be reduced by use of pneumococcal vaccine in the high-risk population.[1617] It requires knowledge of prevalent serotypes and effective vaccination policies at regional and national levels.
Pneumococcal vaccine has been included in national immunization schedules in some Asian countries and recently Pakistan has become one of them. In India, it has yet to become a part of the national immunization program. Besides vaccination, antibiotic prescription policies play a vital role in reducing emergence and spread of drug-resistant phenotypes.
CONCLUSIONS
The present study emphasizes judicious use of antibiotics, routine screening for antimicrobial susceptibility pattern and continued surveillance to monitor existing trends as well as a change in the same for optimal therapeutic outcome. It also highlights the need for vaccination to be included in national program by the policy makers and health care administrators.
Further studies with large sample size are essential to formulate hospital-based strategies and to generalize the findings.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Streptococcus pneumoniae. In: Bennett JE, ed. Mandell, Douglas and Bennett's Principle and Practice of Infectious Diseases Vol Vol. 1. (8th ed). Canada: Elsevier Saunders Publication; 2015. p. :2310-25.
- [Google Scholar]
- Resistant Pneumococcal Infections: The Burden of Disease and Challenges in Monitoring and Controlling Antimicrobial Resistance. Available from: http://www.who.int/csr/resources/publications/drugresist/Pneumonoccal_infections.pdf
- Regional epidemiology of invasive pneumococcal disease in Asian adults: Epidemiology, disease burden, serotype distribution, and antimicrobial resistance patterns and prevention. Int J Infect Dis. 2013;17:e364-73.
- [Google Scholar]
- Epidemiology of Invasive Pneumococcal Disease in Adults in India. 2007. Association of Physicians. Available from: http://www.apiindia.org/pdf/medicine_update_2007/101.pdf
- [Google Scholar]
- Pneumococcal infection in adults: Burden of disease. Clin Microbiol Infect. 2014;20(Suppl 5):45-51.
- [Google Scholar]
- Invasive pneumococcal disease associated with high case fatality in India. J Clin Epidemiol. 2013;66:36-43.
- [Google Scholar]
- Streptococcus pneumoniae: Epidemiology, risk factors, and strategies for prevention. Semin Respir Crit Care Med. 2009;30:189-209.
- [Google Scholar]
- Changing trends in antimicrobial resistance and serotypes of Streptococcus pneumoniae isolates in Asian countries: An Asian Network for Surveillance of Resistant Pathogens (ANSORP) study. Antimicrob Agents Chemother. 2012;56:1418-26.
- [Google Scholar]
- High prevalence of antimicrobial resistance among clinical Streptococcus pneumoniae isolates in Asia (an ANSORP study) Antimicrob Agents Chemother. 2004;48:2101-7.
- [Google Scholar]
- Predominance of ST320 among Streptococcus pneumoniae serotype 19A isolates from 10 Asian countries. J Antimicrob Chemother. 2011;66:1001-4.
- [Google Scholar]
- Gram positive cocci part II: Streptococci, enterococci and the “Streptococcus-like” bacteria. In: Koneman's Color Atlas and Textbook of Diagnostic Microbiology (6th ed). Baltimore: Lippincott Williams and Wilkins; 2006. p. :689-93.
- [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-fourth Informational Supplement M100-S24. Wayne, PA, USA: CLSI; 2014.
- The IndiaCLEN Invasive Bacterial Infection Surveillance (IBIS) Study: The Burden of Vaccine Preventable Illness. Monograph No. 6, December, 1996. Available from: http://www.inclentrust.org/inclen/uploadedbyfck/file/publication/the%20inclen/Annex%201-IBISI%202final.pdf
- [Google Scholar]
- Bacteriological profile of community acquired acute bacterial meningitis: A ten-year retrospective study in a tertiary neurocare centre in South India. Indian J Med Microbiol. 2007;25:108-14.
- [Google Scholar]
- Pneumococcus. In: Paniker CK, ed. Textbook of Microbiology (5th ed). Chennai: Orient Longman Ltd.; 1997. p. :198-203.
- [Google Scholar]
- Invasive pneumococcal infections in Vellore, India: Clinical characteristics and distribution of serotypes. BMC Infect Dis. 2013;13:532.
- [Google Scholar]
- Epidemiology and outcome of invasive pneumococcal disease among adults in Belgium, 2009-2011. Euro Surveill. 2014;19:14-22.
- [Google Scholar]





