Translate this page into:
Subphenotypes of SARS-CoV-2-Associated ARDS Overlap Each Other: A Retrospective Analysis
Address for correspondence: Souvik Maitra, MD, DNB, EDIC, Department of Anaesthesiology, Pain Medicine & Critical Care, Room No: 5013, Teaching Block, Ansari Nagar, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110019, India (e-mail: souvikmaitra@aiims.edu).
-
Received: ,
Accepted: ,
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus-associated pneumonia and acute respiratory distress syndrome (ARDS) were often associated with hyperinflammation and elevation of several serum inflammatory markers but usually less than what is observed in non-coronavirus disease (COVID) ARDS. Elevated inflammatory markers such as C-reactive protein, interleukin (IL)-6, etc., are associated with severe infection. This study identified subphenotypes of COVID-19 ARDS patients by latent profile analysis in a cohort of Indian patients.
Methods
Data of n = 233 adult Indian patients with laboratory-confirmed SARS-CoV-2 infection admitted to a tertiary care teaching hospital were analyzed in this retrospective study. Only patients with acute respiratory failure (defined by partial pressure of oxygen/fraction of inspired oxygen ratio < 200 mm Hg) and chest X-ray showing bilateral infiltrates were included.
Results
The patients' mean (standard deviation) age was 53.3 (14.9) years, and 62% were male. A two subphenotypic model was formulated based on the lowest Bayesian information criterion. Neutrophil-to-lymphocyte ratio and serum IL-6 were latent variables in that model (entropy 0.91). The second phenotype (hyperinflammatory) had lower platelet count (p = 0.02), higher serum creatinine (p = 0.004), higher C-reactive protein (p = 0.001), higher ferritin (p < 0.001), and serum lactate dehydrogenase (p = 0.009). Age-adjusted hospital mortality (p = 0.007), duration of hospital stay (p < 0.001), and duration of intensive care unit stay (p < 0.001) were significantly higher in the second subphenotype.
Conclusion
Two distinct but overlapping subphenotypes were identified in SARS-CoV-2-associated respiratory failure. Hyperinflammatory subphenotype was associated with significantly poor short-term outcomes.
Keywords
ARDS
COVID-19
SARS-CoV-2
latent class
Introduction
Severe infection from the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus can lead to pneumonia and acute respiratory distress syndrome (ARDS).[1] Initial data from China reported that ARDS was present in nearly 42% of patients with coronavirus disease 2019 (COVID-19) pneumonia and carried mortality over 50%.[2] SARS-CoV-2 virus-associated pneumonia and ARDS were often associated with hyperinflammation and elevation of several serum inflammatory markers but usually less than what is observed in non-COVID ARDS.[3] Elevated inflammatory markers such as C-reactive protein (CRP), interleukin (IL)-6, etc., were associated with severe infection.[4]
Despite an objective case definition,[5] ARDS in intensive care units (ICUs) are often underrecognized.[6] Significant clinical heterogeneity in ARDS is well defined as ARDS can be triggered by a myriad of pulmonary and extrapulmonary causes. The severity of ARDS is also variable and classically defined by the degree of hypoxemia, which ranges from mild to severe.[7] Previous studies have found the existence of two distinct subphenotypes in non-COVID ARDS patients.[8] The hyperinflammatory subphenotype of ARDS was associated with elevated inflammatory biomarkers, a higher requirement of vasopressors, and worse clinical outcomes.[8] Identification of subtypes of ARDS is clinically significant as the majority of the successful experimental therapies were ultimately found to be ineffective in the general ARDS population.[7] We hypothesize that severe COVID-19 infection-associated ARDS might have several latent subphenotypes and carry a different prognosis.
Methods
Nucleic acid-based laboratory test-confirmed SARS-CoV-2 infected adult (age > 18 years) patients with acute respiratory failure (defined by partial pressure of oxygen [PaO2]/fraction of inspired oxygen [FiO2] ratio < 200 mm Hg) and chest X-ray showing bilateral infiltrates, admitted in an ICU of a tertiary care teaching hospital, New Delhi, India, were recruited in this study. The data collection period was between May 2020 and October 2020. Strict adherence to the Berlin definition of ARDS[5] was not technically feasible as several patients were managed by oxygen therapy through a high-flow nasal cannula. No formal sample size estimation was performed for this study, and approximately 200 to 250 patients' data were planned to be included in this analysis.
Patients' demographic (age, sex, height, weight), clinical conditions, and preexisting comorbid illnesses were noted. In addition, patients' baseline laboratory parameters that included hematological and biochemical investigations were noted, and all investigations available within the first 24 hours of ICU admission were considered as “baseline.” The respiratory support requirement at the time of ICU admission and clinical outcome (survival or death) at the time of hospital discharge was also noted for all patients.
Results
Data of n = 233 patients with laboratory-confirmed SARS-CoV-2 infection leading to acute hypoxemic respiratory failure (PaO2/FiO2 ratio < 200 mm Hg) were included in this study. The mean (standard deviation) age of the patients was 53.3 (15) years, and 165 of all patients (proportion [95% confidence [CI]] 70.8 [64.7–76.3]) survived hospital discharge. Sixty-two percent of all patients were male. The median (interquartile [IQR]) PaO2/FiO2 ratio was 150 (135–195) mm Hg. Median (IQR) duration of hospital stay and ICU stay were 11 (9–14) and 8 (6–11) days, respectively.
Initially, a two subphenotypic model was constructed with the available inflammatory markers (neutrophil-to-lymphocyte ratio [NLR], lactate dehydrogenase, IL-6, serum ferritin, and CRP). However, the model constructed by IL-6 and NLR had the lowest Akaike information criterion (AIC) (AIC 4,735, entropy 0.90, bootstrap likelihood ratio test p = 0.01, ►Fig. 1). A comparison of baseline variables between subphenotype 1 and subphenotype 2 has been depicted in ►Table 1. Patients belonging to the subphenotype 2 had a lower baseline PaO2/FiO2 ratio, higher CRP, ferritin, and creatinine, and lower platelet count. Male preponderance was also seen in subphenotype 2. However, baseline hemoglobin, total leukocyte count, and international normalized ratio were similar in both the subphenotypes. Unadjusted analyses revealed that the requirement of mechanical ventilation (odds ratio [OR] [95% CI] 6.4 [3.4–11.8], p < 0.001) and hospital mortality (OR [95% CI] 6.2 [3.3–11.6], p < 0.001) were higher in the subphenotype 2. In addition, duration of hospital stay (median [IQR] 11 [9–13] vs. 14 [11–17] days, p < 0.001) and duration of ICU stay (median [IQR] 7 [6–9] vs. 11.5 [8–14] days, p < 0.001) were significantly higher in subphenotype 2.
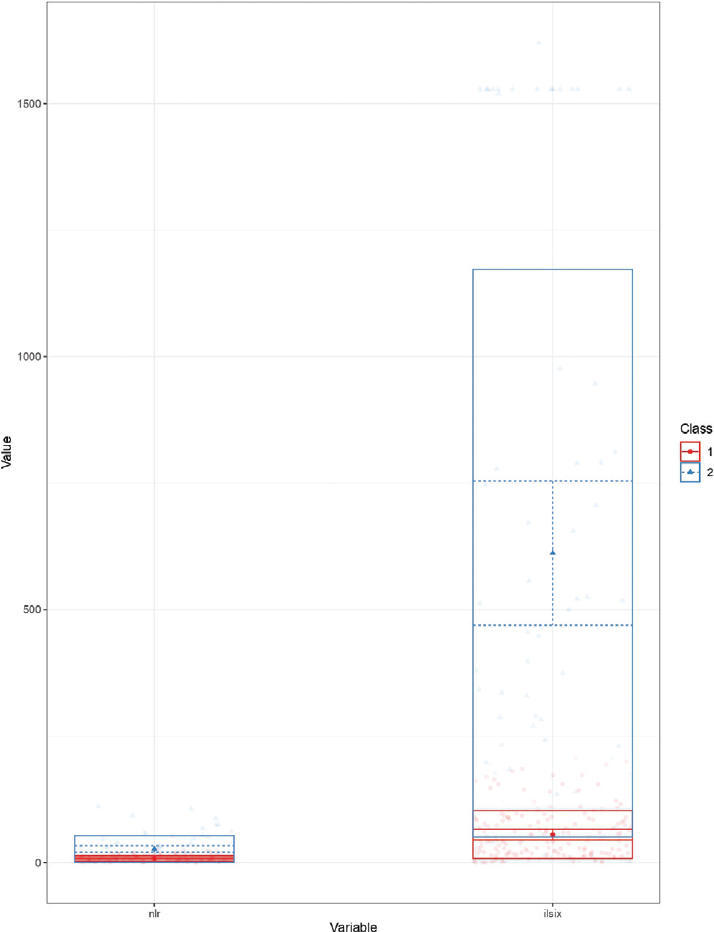
- Profile plot showing distribution of neutrophil-to-lymphocyte ratio and interleukin (IL)-6 in both subphenotypes.
| Variable | All Patients | Subphenotype 1 | Subphenotype 2 | Significance |
|---|---|---|---|---|
| Age | 55 (45–65) | 55 (46–64) | 56.5 (44.3–66.5) | p = 0.44 |
| Sex (male/female) | 145/88 | 100/67 | 45/21 | p = 0.24 |
| Hemoglobin | 11.6 (9.1–13.5) | 11.8 (9.3–13.5) | 11.4 (7.9–13.1) | p = 0.14 |
| TLC | 10300 (6700–15500) | 9900 (6710–15300) | 10590 (6675–17775) | p = 0.44 |
| Platelet | 193 (130–270) | 209 (135–289) | 172 (101–224) | p = 0.005 |
| INR | 1.18 (1.08–1.3) | 1.17 (1.1–1.23) | 1.2 (1–1.5) | p = 0.42 |
| Creatinine | 1.2 (0.8–2.2) | 1.1 (0.8–1.9) | 1.6 (1.04–3.1) | p = 0.004 |
| LDH | 585 (406–808) | 542 (404–739) | 690 (445–937) | p = 0.009 |
| Ferritin | 716 (360–1385) | 585 (329–1130) | 975 (574–2000) | p < 0.001 |
| Bilirubin | 0.6 (0.4–0.95) | 0.6 (0.4–0.9) | 0.63 (0.5–1) | p = 0.24 |
| PaO2/FiO2 ratio | 142 (110–174) | 156 (129–177) | 110 (87–155) | p < 0.001 |
Abbreviations: FiO2, fraction of inspired oxygen; INR, international normalized ratio; LDH, lactate dehydrogenase; PaO2, partial pressure of oxygen; TLC, total leukocyte count.
After adjustment of age and baseline PaO2/FiO2 ratio, subphenotype 2 had significantly higher mortality (OR [95% CI] 3.1 (1.4–6.9], p = 0.007) and requirement of mechanical ventilation (OR [95% CI] 3 [1.1–8.1], p = 0.029). In addition, duration of hospital stay (t = 3.51, p > 0.001) and duration of ICU stay (t = 4.12, p < 0.001) were also significantly higher in subphenotype 2, even after adjustment of age and baseline PaO2/FiO2.
Discussion
This study identified two subphenotypes of COVID-19 infection-associated ARDS and subphenotype 2, which were associated with “hyperinflammation” and poor clinical outcomes.
Classically, ARDS is a heterogeneous disorder, and there are significant pathophysiological variations between pulmonary and extrapulmonary ARDS.[9] Subphenotypes of ARDS were identified from secondary analyses of various randomized controlled trials. Calfee et al identified two subphenotypes of ARDS; the hyperinflammatory subphenotype was associated with the elevation of inflammatory biomarkers, acidosis, and sepsis. Clinical outcome was also worse in that subphenotype.[8] Our findings are also quite similar to the previous data as we were also able to identify two subphenotypes, and poor clinical outcome was evident with hyperinflammation. Similar subphenotypes were identified in COVID-19 ARDS patients also.[10]
Identification of subphenotypes in COVID-19 pneumonia/ARDS patients has several clinical implications. As patients with elevated inflammatory biomarkers have a worse clinical outcome and higher requirements for mechanical ventilation, they should be managed in a well-equipped center. During the time of the pandemic, the categorization of the patients is important for proper resource utilization.
Identification of subphenotypes of COVID-19 ARDS has several therapeutic implications. First, future randomized controlled trials can be designed for a particular subphenotype to avoid heterogeneity in the clinical status of the participants. Apart from this, anti-inflammatory drugs are the mainstay of treatment in COVID-19 pneumonia patients, and they may be more useful in patients with a hyperinflammatory state.
Limitations
Our study has several limitations. Most importantly, the sample size of our study was limited and strict adherence to the Berlin definition was not practically feasible. Furthermore, we conducted this study during the so-called “first wave” of the COVID-19 pandemic in New Delhi. As the COVID-19 pandemic is evolving, variants of the SARS-CoV-2 virus also evolve, and disease severity also changes. Hence, there is some probability that the study findings may not apply to other geographical locations or when other virus variants are causative agents.
Conclusion
Two distinct but overlapping subphenotypes were identified in SARS-CoV-2-associated respiratory failure. In addition, the hyperinflammatory subphenotype was associated with a significantly poor short-term outcome even after adjustment of baseline disease severity.
Note
Abstract of this manuscript was accepted for presentation in ESICM Lives 2022 held in Paris from 22nd to 26th October.
Conflict of Interest
None declared.
Funding
None.
References
- COVID-19 acute respiratory distress syndrome (ARDS): clinical features and differences from typical pre-COVID-19 ARDS. Med J Aust. 2020;213(02):54-56.e1.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(07):934-943.
- [CrossRef] [PubMed] [Google Scholar]
- Cytokine levels in critically ill patients with COVID-19 and other conditions. JAMA. 2020;324(15):1565-1567.
- [CrossRef] [PubMed] [Google Scholar]
- Association of inflammatory markers with the severity of COVID-19: a meta-analysis. Int J Infect Dis. 2020;96:467-474.
- [CrossRef] [PubMed] [Google Scholar]
- ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526-2533.
- [CrossRef] [Google Scholar]
- LUNG SAFE Investigators, ESICM Trials Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(08):788-800.
- [CrossRef] [PubMed] [Google Scholar]
- ARDS subphenotypes: understanding a heterogeneous syndrome. Crit Care. 2020;24(01):102.
- [CrossRef] [PubMed] [Google Scholar]
- NHLBI ARDS Network. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2(08):611-620.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and biological heterogeneity in acute respiratory distress syndrome: direct versus indirect lung injury. Clin Chest Med. 2014;35(04):639-653.
- [CrossRef] [PubMed] [Google Scholar]
- Latent class analysis reveals COVID-19-related acute respiratory distress syndrome subgroups with differential responses to corticosteroids. Am J Respir Crit Care Med. 2021;204(11):1274-1285.
- [CrossRef] [PubMed] [Google Scholar]






