Translate this page into:
The role of discriminant functions in screening beta thalassemia trait and iron deficiency anemia among laboratory samples
Address for correspondence: Dr. Jyoti Kini, Department of Pathology, Kasturba Medical College, Mangalore (Manipal University), Light House Hill Road, Mangalore, Karnataka, India. E-mail: kinijyoti@gmail.com
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
INTRODUCTION:
Most important differential diagnosis for microcytosis and hypochromia is beta thalassemia trait (BTT) and iron deficiency anemia.
AIM:
To study the utility of discriminant functions (DFs) and red cell indices in distinguishing BTT and iron deficiency anemia.
METHODS:
The study is observational (cross sectional). A total of 350 patients, 43 BTT, and 307 iron-deficiency anemia reflecting actual disease prevalence were included. Their complete red blood cell parameters, hemoglobin A2, and serum ferritin level wherever required were obtained. Receiver operator characteristic curve was drawn for each DF and results compared with other studies.
RESULTS:
Among the six DFs, the highest sensitivity (97.7%) and specificity (98.6%) was shown, respectively, by Shine and Lal (S and L) and England and Fraser index (E and F) in identifying cases of BTT. Youden index of the Mentzer index (MI) was the highest (69.0) and S and L, the lowest (13.2) indicating MI to be the most efficient and the S and L, the least in differentiating the two entities. Red cell distribution width index (RDWI) showed the highest accuracy (91.6%), whereas S and L showed the least accuracy (29.6%).
CONCLUSION:
MI was the most efficient in discriminating BTT from iron deficiency anemia (IDA). RDWI stands to be the most accurate. S and L could at best be used as screening tool rather than DF. No study except one agreed with us because convenient sampling used in other studies generated bias in their results. Statistically, this study bears far more relevance than other studies because the sample distribution reflects the prevalence of IDA and BTT in the community.
Keywords
Discriminant functions
England and Frazer index
Mentzer index
microcytosis
receiver operator characteristic curve
Shine and Lal index
Youden index
Introduction
Microcytic hypochromic anemia, especially in the Indian context, usually is due to beta thalassemia trait (BTT) or iron deficiency anemia (IDA). IDA is known to be the most common nutritional disorder in the world.[1] Screening of thalassemia minor is the only method to prevent the occurrence of homozygotes in the society. The definitive diagnosis is made by iron profile for IDA and high performance liquid chromatography (HPLC) for BTT. These are expensive and require sophisticated analyzers. The present study was conducted to prospectively assess the discriminant efficiency and usefulness of selected red blood cell (RBC) indices, i.e., RBC count, mean corpuscular volume (MCV), red cell distribution width (RDW), and six discriminant functions (DFs) that are the Shine and Lal (S and L)[2] index, Srivastava[3] index (SI), England and Fraser[4](E and F) index Ricerca index[5](RI), Mentzer index[6](MI), and RDW index (RDWI).[7] In the present study, these indexes were compared along with construction of the receiver operator characteristic (ROC) curve, and therefore, the most efficient DF, proposed.
Materials and Methods
Lab procedures, instruments and discriminant functions
Beckman Coulter LH 780, automated 5-part differential cell counter analyzed the blood samples. LH 780 was calibrated half yearly using manufacturer's calibrator. A 3-level internal quality control using manufacturer's material and preserved blood of patients ensured precision. An external quality assurance scheme is used quarterly to check instrument performance. BIORAD D-10 analyzer (Biorad Laboratories, Hercules, CA, USA), a cation exchange automated HPLC system analyzed samples for Hb variant. HbA2 calibrator and two levels of controls were analyzed at the start of each run. HbA2 between 1.5% and 3.5% was accepted as normal.[8] HbA2 >3.5% was considered as BTT if no other abnormal Hb was found. Electrochemiluminescence immunoassay using Cobas e411 immunoassay analyzer estimated serum ferritin, which below 15 and 12 ng/mL for males and females, respectively, usually indicated IDA.[9]
Study design
The observational study was performed for 2 years from August 1, 2012 to August 1, 2014. Requests for complete hemogram came from various departments viz. medicine, obstetrics, surgery, etc. A report suggestive of microcytic hypochromic anemia was delivered on blood samples which met the following criteria:
-
MCV <80 fL[9]
-
Hemoglobin (Hb) <13 g/dl for males and <12 g/dl for females
-
Peripheral Blood Smear (PBS) picture of microcytosis and hypochromia.
Many of these patients were referred further to the laboratory for HPLC. Samples suggested BTT by HPLC were not further investigated. Those that had no Hb variant (normal) were further analyzed for serum ferritin levels, some ordered by the investigating physician and many paid by the authors. Samples with low serum ferritin levels were deemed as IDA.
Opportunity sampling method was used without any bias regarding their place of birth, profession, community, marital status, general health, and clinical details. Patients with liver or renal diseases, long standing chronic illness (autoimmune or infective), patients hospitalized for any acute illness or those having received whole blood/packed red cell transfusion or iron therapy were excluded. The study was approved by the Institutional Ethics Committee.
Study population
The sample size was calculated by:

Where, n = desired sample size, z = standard normal deviate usually at 1.96, P = prevalence of the disease, q = 1.0–p, d = degree of accuracy desired that is 0.05. The prevalence of IDA is 24.7% in males and 56%–57% in females.[10] The prevalence of BTT is 1%–3% in southern India.[11] Thus, the sample size for IDA should have been approximately 370 and BTT, 45.
The sample size was 350 that included 223 females (63.6%) and 127 males (36.4%), 43 were BTT and 307, IDA.
Laboratory data and statistical analysis
The patient data entered in Microsoft Excel was statistically analyzed by SPSS version 16.0 (SPSS Inc., Chicago, IL, USA).
Receiver operator characteristic curves and Youden index
The ROC curves were plotted to better compare the efficacies of the DFs to distinguish BTT from IDA and derive new cut-offs to maximize the sensitivity and specificity for each DF. YI = (sensitivity + specificity)−100. The DF with highest area under the ROC curve and maximum YI is considered the best.
Results
The males predominated in the prevalence of BTT (26/43, 60.5%) compared to the females (17/43, 39.5%) whereas in case of IDA, the females (211/307, 68.6%) compared to the males (96/307, 31.4%) prevailed and was statistically significant. The difference in the mean values of RBC count, Hb, HCT, MCV, MCH, RDW, and RDW-standard deviation (RDW-SD) was highly significant to distinguish between BTT and IDA (P < 0.001), whereas the MCHC remained nondiscriminant (P > 0.05) [Table 1]. All DFs could significantly distinguish between IDA and BTT except S and L index [Table 2]. The RDWI showed the highest area under the ROC curve (area under the curve [AUC]) i.e., 0.929 cm2 and the S and L index, the lowest (0.815 cm2) [Table 3]. The ROC curve also helped suggest new cut-off values of all indexes that performed with better sensitivity, specificity, and YI, when compared with the standard cut-off values [Table 4]. Figures 1 and 2 show the ROC curves to visually compare the AUC.

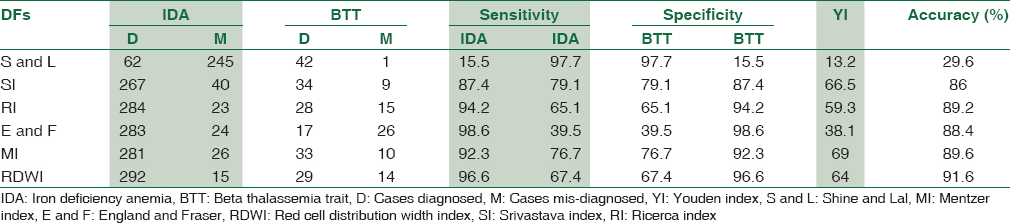


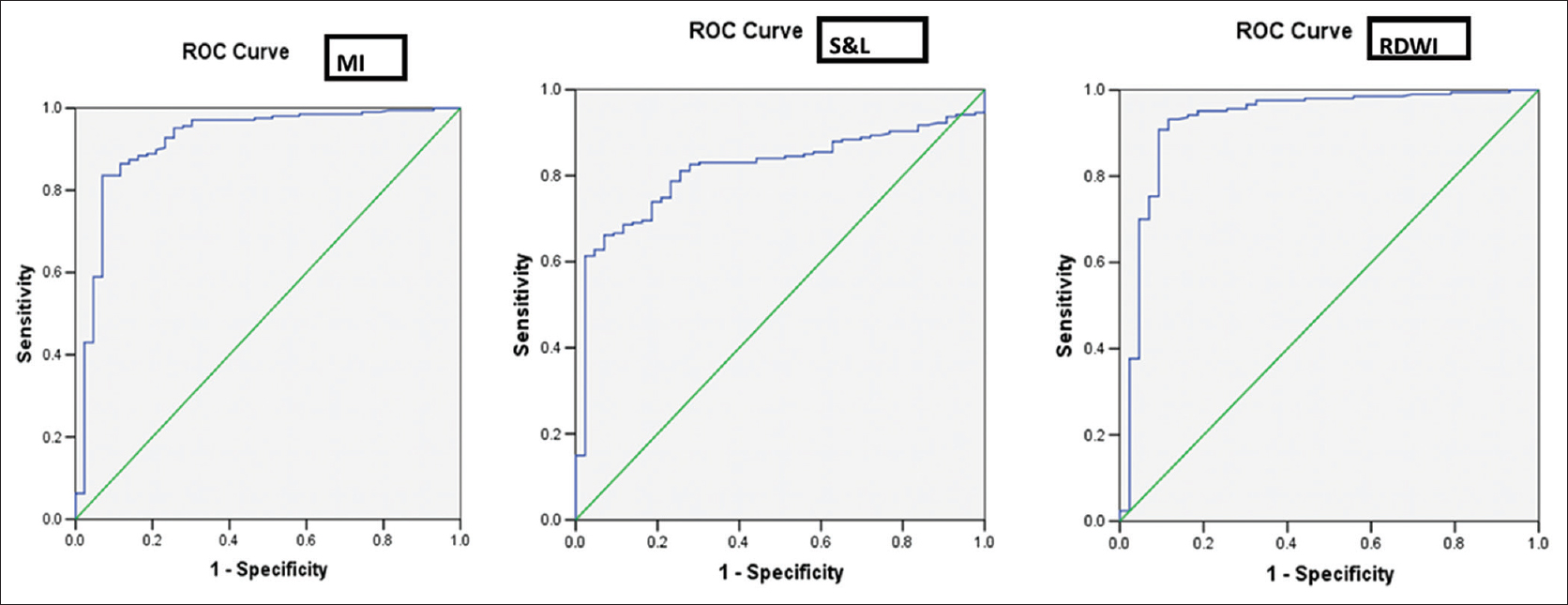
- Receiver operator characteristic curves of Mentzer index, Shine and Lal index and red cell distribution width index
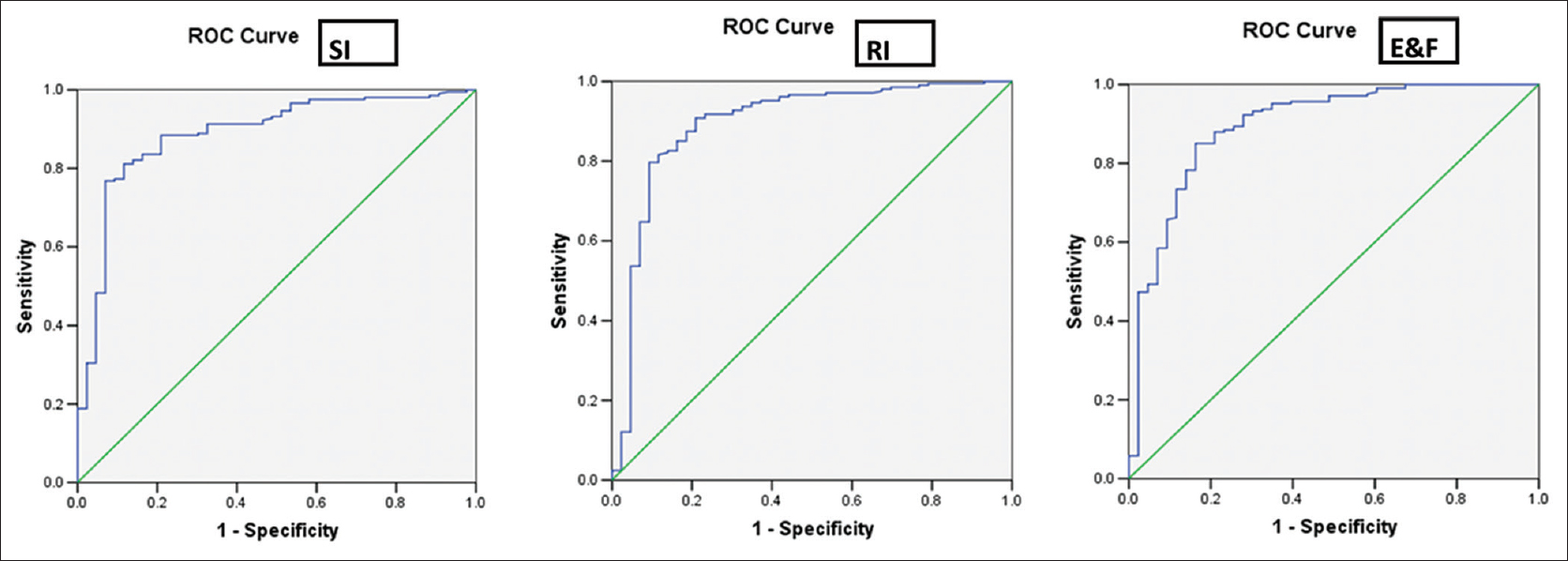
- Receiver operator characteristic curves of Srivastava index, Ricerca index and England and Fraser index
Discussion
Three investigators, Klee et al.,[7] Demir et al.,[12] and Beyan et al.[13] discriminated between IDA and BTT best with the RBC count, the latter two obtaining a Youden index (YI) of 82%[12] and 73.7,[13] respectively. In the present study, the RBC count among the IDA cases showed a range of 1.04–6.12 × 1012/L (mean 3.98, SD ± 0.649), whereas in BTT cases, it remained in the range of 3.02–6.80 × 1012/L (mean 5.22, SD ± 0.716). The YI of the present study was 68.6, much lower than that of 93.5, derived by Kotwal et al.[14]
Kotwal et al. proved MCV as an effective discrimination index for IDA and BTT at a cut-off value of ≤76 fL for Indian population.[14] Other investigators, Ghosh et al.[15] Pearson et al.,[16] and Lafferty et al.[17] derived cut-offs of <75 fL, <80 fL, and <72 fL, respectively, for other populations. Milunsky et al. observed a very high probability of BTT showing MCV <60 fL.[18] MCV had the lowest sensitivity (81.3%) among all the RBC indices in detecting BTT.[19] In the present study, the MCV among the IDA cases was 69.5 fL ± 6.5, whereas in BTT cases, it remained at 62.6 fL ± 4.5. The sensitivity, specificity of MCV at a cut-off range of 76 fL in BTT, was found to be 97.7% and 17.4% respectively. Thus, the YI observed in the present study is 31.2, in the study by Kotwal et al. was 51.3.[14] At 78 fL, d’Onofrio et al. could correctly identify 70.8% (206/291) patients,[20] whereas the present study, much less, 31.2% (109/350).
Kotwal et al.[15] at cutoff ≤18, Bessman et al.[21] and Robert et al.[22] observed RDW as a good discrimination index for IDA and BTT. Lafferty et al.,[17] Flynn et al.,[23] Cesanan et al.,[24] and Miguel et al.[25] in contrast, concluded RDW is inadequate as a discriminant index. In the present study, the RDW among the IDA cases was 19.2 ± 4.1, whereas in BTT, it remained at 16.8 ± 2.7. The sensitivity, specificity of RDW at a cut-off range of ≤18 remained 55.6%, 83.7%, respectively, in IDA (reversed in BTT); YI was 39.3.
Lee et al. correctly identified 99% of uncomplicated BTT with the help of E and F.[26] d’Onofrio et al. could correctly diagnose 260/291 cases with E and F and so, the discriminant efficiency remained 89.3%.[20] In the present study, the sensitivity and specificity in BTT cases were 39.5% and 98.6%, respectively, the YI was 38.1. Hence, in the present study, E and F index showed unsatisfactory results consistent with the study by Yeo et al.[27]. At the new cut-off of 11.06 derived from the ROC, the sensitivity, specificity, and YI (68.7) were considerably increased.
Okan et al. in their study proved S and L as the best discrimination index with highest YI.[28] Similarly, Yeo et al. reported a 38.7% increase in accuracy and 31.1% decrease in confirmatory testing among pregnant patients.[27] Niazi et al. reported accuracy, specificity, sensitivity, and YI of 72.43 (lowest among all DFs used in their study), 100%, 72% (lowest but for SI) and 72, respectively.[29] The accuracy, in the present study, S and L index showed the highest sensitivity (97.7%) in detecting BTT but with specificity of 15.5%, it performed as a poor DF with a lowest YI of 13.2. However, going by the ROC, at a cutoff of 874.4, the specificity and sensitivity came to 81.2% and 74.4% bringing up the YI to 55.6. We conclude that S and L index is sensitive but can hardly fulfill the criterion of a DF.
DeMaeyer et al. observed the Srivastava index (SI) to be valuable and convenient in distinguishing IDA from BTT.[30] d’Onofrio et al. could categorize 83.5% (243/291) IDA and BTT patients.[20] Likewise, the accuracy found in the present study was 86% (301/350). In the present study, sensitivity and specificity of 79.1% and 87.4% and a YI of 66.5 could be realized. At a new cutoff of 4.63 (from ROC curve), sensitivity and specificity of 86% and 82.1%, respectively, was accomplished with YI of 68.1.
In a study by d’Onofrio et al., RI showed a discriminant efficiency of 85.6%.[20] In the present study, RI could accurately diagnose 89.2% (312/350) cases and showed a YI of 59.3. At the new cut-off of 3.8 (from ROC curve), the sensitivity, specificity, and YI increased to 79.7%, 90.7%, and 70.4, respectively.
Ehsani et al. observed MI as the best discriminatory index with the highest YI (90.1) with accuracy of 97.71% (269/284)[31] compared to 89.6% (314/350) obtained in the present study, and 89% (259/291) achieved by d’Onofrio et al. at a cut-off of 14.[20] Batebi et al. obtained sensitivity, specificity, and YI of 86.3%, 85.4%, and 71.7, respectively at a cut-off of 13.[19] Niazi et al. obtained accuracy, sensitivity, specificity, and YI of 86.85% (2nd to RDWI), 89%, 81%, and 70, respectively.[29] In the present study, MI achieved sensitivity, specificity, and YI of 92.3%, 76.7%, and 69 respectively. Our YI was similar to that of Batebi et al.[19] and Niazi[29] et al. though falling short of that of Ehsani et al.[30] At the new cut-off of 14.07 derived from the ROC, the sensitivity and specificity were 86% and 87.4%, marginally augmenting the YI to 73.4.
RDWI was second to RBC count in discriminating BTT from IDA with YIs of 80 and 82, respectively, and accuracy of 91.6% and 90%, respectively.[12] Nesa et al. found a sensitivity, specificity, and YI of 80.7%, 84.7%, and 65.4, respectively, using the conventional cut-off value of 220.[32] In the present study, the RDWI showed the best discriminatory efficiency enclosing the highest area (0.929 cm2) under the ROC curve among the studied DFs with sensitivity and specificity of 96.6% and 67.4% and YI of 64. Niazi et al. also proved it to be the best DF with accuracy, sensitivity, specificity, and YI of 88.14%, 91%, 81%, and 72, respectively.[29] At the revised cut-off of 231.6, the sensitivity and specificity stood at 93.2% and 88.4%, respectively, with a YI of 81.6. Since 230.5 is much higher than 220, reservations may arise regarding its acceptance.
Review of the recent studies on this topic recounted both concurrence and conflict with our study. Adlekha et al. in 2014 also found RDWI to exhibit the highest YI similar to our study followed by the discriminant score, a complex formula that varies between males and females.[33] Ntaios et al. in 2007 used Gaussian curves to prove the supremacy of G and K index with a YI of 70.86% and accuracy of 80.12% followed by the E and F.[34] Trivedi and Shah in 2010 studied discriminant efficiency in 81 IDA and 135 BTT patients that divulged the preeminence of a new DF, the RDW-SD with accuracy of 92.13%, and YI of 84.93 at a cut-off of 46 fL (IDA > 46, BTT < 46) followed by the RBC count with YI of 72.5.[35]
In the recent past, improved hematology analyzers enabled advanced cell data to be displayed, though at higher costs. Pioneering among these was d’Onofrio et al. who invented the formula of the ratio of microcytic(M) to hypochromic(H) cells that displayed better sensitivity and specificity than the traditional DFs.[20] This was further improved by Urrechaga et al. in 2008 who propounded the formula M-H >11.5 to be indicative of BTT and <11.5, for IDA.[36] Shoorl et al. in 2012 used microcytic and hypochromic cells percentage along with reticulocyte count and hemoglobinization of reticulocytes and RBCs to invent a complex algorithm to differentiate BTT and IDA with superior precision.[37] However, this requires analyzers to be able to spew all the required data which are not routinely required and the machine's computer be fitted with the algorithm and thus, it will escalate the costs of routine counts which was the main drawback that prevented us to perform HPLC to detect HbA2 in the first place.
Studies that split patient set into three subgroups viz. BTT, BTT with IDA, and IDA were ignored in the discussion. To make a DF perform in such a kind of approach would require two cut-offs to be determined along with an algorithmic approach to discretely identify the three groups, in the absence of any other laboratory tests. However, this approach was not followed in these studies; rather a cutoff was established and sensitivity and specificity were individually determined in each group. Obviously, a single DF with a sole cut-off value will not be able to distinguish three groups. The DFs are designed to differentiate BTT from IDA. DFs used on RBC indices of patients suffering concomitantly from BTT and IDA would either identify them as BTT or IDA and either of these diagnoses would be wrong. Ergo, ’IDA with BTT’ is a category best left taboo when efficiency of DFs is being investigated. Perhaps, a different algorithm is required to resolve this problem.
BTT state entails some degree of ineffective erythropoiesis. The hepcidin level is undetectable/very low as the erythroid precursors release growth differentiation factor 15 and twisted granulation protein 1 that inhibit hepcidin synthesis. This, in turn causes increase in iron absorption.[38] Moreover, this part of India where the study was conducted is populated by an overwhelming majority of nonvegetarians. Thus, we may not have encountered the perplexity of “BTT with IDA” prevalent elsewhere in India.
Limitations and strengths of the current study
Opportunity sampling method was used in this study. It ignores those IDA as well as BTT patients for whom HPLC was not asked. In addition, the BTT cases did not have their serum ferritin levels estimated. Thus, cases of BTT concomitant with IDA could not be identified, though arguably, BTT with IDA is less likely to arise.
Most studies used convenient sampling in that IDA cases were very few compared to a very large number BTT cases. Concluding the discriminant efficiency of any function with such a study design probably suffers from compromise in the epidemiological value of a DF. From the diagnostic point of view of any laboratory, a DF which identifies both BTT and IDA with accuracy approaching 100% would be ideal. That S and L index which does identify almost all BTT cases and in the process, apportions a vast majority of the IDA cases in the BTT group has been overlooked by most studies except that by d’Onofrio et al.[20] That S and L index can be used as a screening tool and not a discriminant index has been alluded to only in their study.[20] The sample distribution of any comparative study testing a DF should reflect the existing prevalence of both diseases, e.g., Niazi et al. had 223 BTT cases and just 89 IDA or non-BTT cases.[29] The YI determined from such sample distribution is not representative of the community since IDA is much more prevalent than BTT. Thus, all the studies compared here do have noticeable differences from our study. None bothered to comply their design with the current prevalence of IDA and BTT.
Conclusion
S and L remained highly sensitive (97.7%) and E and F, highly specific (98.6%) in diagnosing BTT. The YI of MI was highest (69.0) at the conventional cut-off, while S and L, the lowest (13.2). RDWI showed the highest accuracy (91.6%), whereas the S and L, the least (29.6%). Thus, while MI was more efficient in discriminating between BTT and IDA, RDWI was the most accurate, an apparent statistical paradox brought about by the skewed sample distribution. RDWI had the highest area under the ROC curve, so potentially it has the best discriminant efficiency and thus at a revised cut-off value of 231.6, it reigned supreme in accuracy, sensitivity, and specificity. S and L index with its incredulously low specificity and accuracy and incredibly high sensitivity, may however be used as a screening index rather than discrimination index. The revised cutoff values for all DFs, as suggested by the ROC must be seriously contemplated for use particularly in the subcontinent, as it considerably enhanced the discriminatory potential.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Iron. In: Garrow JS, James WP, Ralph A, eds. Human Nutrition and Dietetics (10th ed). Edinburgh: Churchill-Livingstone; 2000. p. :177-92.
- [Google Scholar]
- Srivastava PC Differentiation of thalassaemia minor from iron deficiency. Lancet. 1973;2:154-5.
- [Google Scholar]
- Differentiation of iron deficiency from thalassaemia trait: A new approach. Haematologica. 1987;72:409-13.
- [Google Scholar]
- Differentiation of iron deficiency from thalassaemia trait by routine blood-count. Lancet. 1973;1:449-52.
- [Google Scholar]
- Routine erythrocyte measurements in diagnosis of iron-deficiency anemia and thalassemia minor. Am J Clin Pathol. 1976;66:870-7.
- [Google Scholar]
- Henry's Clinical Diagnosis and Management by Laboratory Methods 2001:542-71.
- National Family Health Survey 3. India 2005-06; International Institute of Population Sciences, Mumbai, India and O RC Macro, Calverton, Maryland, USA 2007 October
- Most reliable indices in differentiation between thalassemia trait and iron deficiency anemia. Pediatr Int. 2002;44:612-6.
- [Google Scholar]
- Predictive value of discrimination indices in differential diagnosis of iron deficiency anemia and beta-thalassemia trait. Eur J Haematol. 2007;78:524-6.
- [Google Scholar]
- Erythrocyte indices for discriminating thalassaemic and non-thalassaemic microcytosis in Indians. Natl Med J India. 1999;12:266-7.
- [Google Scholar]
- Evaluation of a prenatal screening procedure for beta-thalassaemia carriers in a Chinese population based on the mean corpuscular volume (MCV) Prenat Diagn. 1985;5:59-65.
- [Google Scholar]
- Screening for thalassemia trait by electronic measurement of mean corpuscular volume. N Engl J Med. 1973;288:351-3.
- [Google Scholar]
- The evaluation of various mathematical RBC indices and their efficacy in discriminating between thalassemic and non-thalassemic microcytosis. Am J Clin Pathol. 1996;106:201-5.
- [Google Scholar]
- Genetic Disorders and the Fetus: Diagnosis, Prevention, and Treatment (4th ed). Baltimore, MD: Johns Hopkins University Press; 1998. p. :673.
- Discrimination of beta-thalassemia minor and iron deficiency anemia by screening test for red blood cell indices. Turk J Med Sci. 2012;42:275-80.
- [Google Scholar]
- Automated measurement of red blood cell microcytosis and hypochromia in iron deficiency and beta-thalassemia trait. Arch Pathol Lab Med. 1992;116:84-9.
- [Google Scholar]
- Improved classification of anemias by MCV and RDW. Am J Clin Pathol. 1983;80:322-6.
- [Google Scholar]
- Red blood cell distribution width index in some hematologic diseases. Am J Clin Pathol. 1985;83:222-6.
- [Google Scholar]
- Limitations of red blood cell distribution width (RDW) in evaluation of microcytosis. Am J Clin Pathol. 1986;85:445-9.
- [Google Scholar]
- Relevance of red cell distribution width (RDW) in the differential diagnosis of microcytic anaemias. Clin Lab Haematol. 1991;13:141-51.
- [Google Scholar]
- Red cell distribution width analysis in differentiation between iron deficiency and thalassemia minor. Acta Haematol. 1988;80:59.
- [Google Scholar]
- Microcytosis and the anemias associated with impaired haemoglobin synthesis. In: Greer JP, Foerster J, Lukens JN, eds. Wintrobe's Clinical Hematology Vol 1. (9th ed). Philadelphia, PA: Lippincott Williams & Wilkins; 1993. p. :791-807.
- [Google Scholar]
- The role of discriminant functions in screening for beta-thalassaemia traits during pregnancy. Singapore Med J. 1995;36:615-8.
- [Google Scholar]
- Red cell indices and functions differentiating patients with the beta thalassaemia trait from those with iron deficiency anaemia. J Int Med Res. 2009;37:25-30.
- [Google Scholar]
- Usefulness of red cell indices in differentiating microcytic hypochromic anemias. Gomal J Med Sci. 2010;8:125-9.
- [Google Scholar]
- Preventing and Controlling Iron Deficiency Anemia Through Primary Health Care. Geneva: WHO; 1989. p. :26-7.
- A new index for discrimination between iron deficiency anemia and beta-thalassemia minor: Results in 284 patients. Pak J Biol Sci. 2009;1(12):473-5.
- [Google Scholar]
- RDWI is better discriminant than RDW in differentiation of iron deficiency anemia and beta thalassemia trait. Bangladesh J Child Health. 2009;33:100-3.
- [Google Scholar]
- Screening of β-thalassemia trait by means of red cell indices and derived formulae. Med J DY Patil Univ. 2013;6:71-4.
- [Google Scholar]
- Discrimination indices as screening tests for beta-thalassemic trait. Ann Hematol. 2007;86:487-91.
- [Google Scholar]
- Discriminant functions in distinguishing beta thalassemia trait and iron deficiency Anemia: The value of the RDW-SD. Internet J Hematol. 2010;7:1-6.
- [Google Scholar]
- Discriminant value of % microcytic/% hypochromic ratio in the differential diagnosis of microcytic anemia. Clin Chem Lab Med. 2008;46:1752-8.
- [Google Scholar]
- Efficacy of advanced discriminating algorithms for screening on iron-deficiency anemia and ß-thalassemia trait: A multicenter evaluation. Am J Clin Pathol. 2012;138:300-4.
- [Google Scholar]
- Iron metabolism, iron deficiency and disorders of haem synthesis. In: Hoffbrand AV, Catovsky D, Tuddenham EG, Green AR, eds. Postgraduate Hematology (6th ed). New York: Wiley-Blackwell; 2011. p. :26-46.
- [Google Scholar]





