Translate this page into:
Twin Malignancy of Acute Myeloid Leukemia and Multiple Myeloma in a Chemotherapy-Naïve Patient: A Rare Occurrence
Address for correspondence: Shuchismita, MBBS, MD, Department of Hematology, Indira Gandhi Institute of Medical Sciences, Patna, 800014, Bihar, India (e-mail: shuchi.smita123@gmail.com).
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Synchronous diagnosis of acute myeloid leukemia (AML) and multiple myeloma in chemotherapy-naïve patients is a rare event and poses a serious therapeutic challenge as it imparts a poor prognosis. We report a case of concurrent AML with multiple myeloma in a 44-year-old male along with a PUBMED-based research of previously reported similar cases in published literature.
Keywords
acute myeloid leukemia
chemotherapy
multiple myeloma
naïve
simultaneous
Introduction
Multiple myeloma (MM) and acute myeloid leukemia (AML) may usually develop in the same patient but they are generally seen in MM patients receiving chemotherapy and in due course of treatment AML develops.[1] However, simultaneous occurrence of MM and acute leukemia on presentation in chemotherapy-naïve patients is rare, with only a 25 cases reported in the literature so far.[2,3] Herein, we are reporting such a case of dual malignancies in a 44-year-old male who was not exposed to any chemotherapy or radiotherapy prior to presentation.
Case Report
A 44-year-old male patient presented with severe generalized weakness, fever, persistent dry cough, and low back pain for 2 months. He had also a past history of repeated blood transfusions for prolonged anemia and generalized weakness. There was no prior history of any long-term medications.
On clinical examination, pallor was present. Abdomen palpation revealed moderate hepatosplenomegaly. Liver function tests showed reverse A:G ratio. Kidney function tests showed increased blood urea and serum creatinine levels.
Complete blood count revealed hemoglobin −6.2 gm/dL and white blood cell count of 44,000/cumm with the presence of 94% blasts, few demonstrating Auer rods. Platelet count was reduced (53,000/cumm; ►Fig. 1A) Bone marrow aspiration was performed that was hypercellular for age with diffuse infiltration by blasts leading to marked suppression of trilineage hematopoiesis with increased plasma cells (10%; ►Fig. 1B). Bone marrow biopsy (done using Hammersmith protocol) revealed presence of few plasma cells that were positive for CD38, CD138, and kappa confirming presence of simultaneous monoclonal gammopathy (Immunohistochemistry was performed manually and antigens were retrieved by heat induced method.) (►Fig. 1C and D). Due to deranged renal function tests, serum protein electrophoresis was ordered that shows presence of M spike (2.2 g/dL) in beta-2 globulin region which turned out to be immunoglobulin A (IgA) kappa on immunofixation confirming monoclonal gammopathy (►Fig. 2).

- (A) Peripheral blood smear showing presence of blasts with Auer rods (Leishman stain; 100 ×). Inset shows myeloperoxidase (MPO)-positive blasts (MPO stain;100 ×). (B) Bone marrow aspirate showing infiltration by blasts with increased plasma cells (Leishman stain; 100 ×). Inset shows the same. (C) Immunohistochemistry (IHC) microphotograph showing membranous CD138 positivity (DAB, 100 ×). (D) IHC microphotograph showing membranous CD38 positivity. Inset shows Kappa restriction (DAB;100 ×).
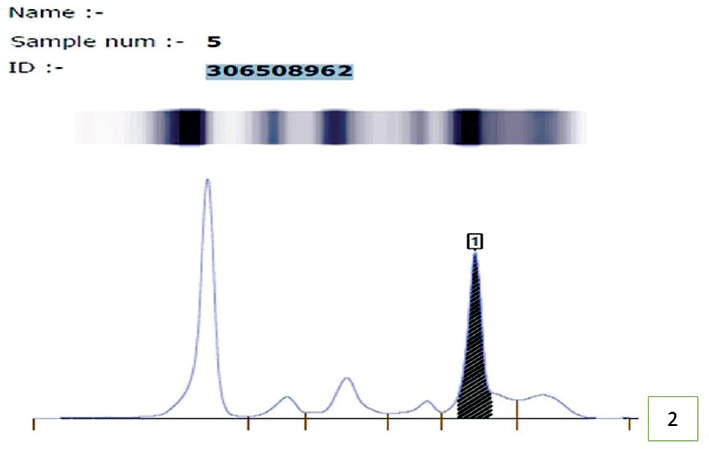
- Serum protein electrophoresis showing M-spike in gamma globulin region suggesting monoclonal gammopathy.
Special stain with myeloperoxidase (MPO) was positive. Immunophenotyping on peripheral blood sample revealed a CD45 positive dim population (94% of the total) of blasts; low side scatter that was positive for CD117, CD13, CD33, and MPO; and negative for CD34, human leukocyte antigen-DR isotype (HLA-DR), cytoplasmic CD3, CD7, Tdt, CD19, CD20, and CD22, thus confirming the myeloid lineage of blasts. Overall morphological and flow cytometric findings were consistent with acute myeloid leukemia without maturation (AML-M1; ►Fig. 3A–E). Thus, it was confirmed that patient had synchronous AML with monoclonal gammopathy.

- (A–C) Immunophenotyping showing positivity for CD 45, cytoplasmic myeloperoxidase (MPO), CD117, CD13, and CD33. (D–E) Immunophenotyping revealing CD34, Tdt, and CD22-negative blast population.
Discussion
The association of AML with MM is seen as a complication of chemotherapy, but it may also occur in the absence of this treatment. This synchronous occurrence of AML and MM in the same patient without previous exposure to chemotherapy is a very rare event. About 5 to 10% of AML cases are seen associated with plasmacytosis; thus, it is extremely important to rule out its neoplastic nature by carrying out MM workup.[3] Secondly, none of the hematological malignancies should be therapy related to fulfill the criteria for the twin diagnosis of AML and MM. Several pathophysiological mechanisms have been proposed to explain the simultaneous occurrence of these two malignancies. These include a disorder of multipotent stem cells exposure to common environmental risk factors and repeated infections in a patient of MM, which might result in the development of a leukemic clone. Another explanation may be that MM is a slowly evolving disease with a resultant decrease in immune surveillance and incipient leukemic clones might escape this immune surveillance concurrently.[4,5]
A PUBMED database search was made for synchronous occurrence of AML with MM from 1970 to 2021 and data was analyzed[1,4-24] (►Table 1).
| Name of the study and year | Age (years) | Sex (M/F) | Subtype of AML (FAB) | Type of paraprotein | Karyotype | Survival from diagnosis |
|---|---|---|---|---|---|---|
| Thijs et al (1970)[1] | 47 | F | AML | IgA/κ | ND | 4 months |
| Taddelnl and Schrader (1972)[4] | 73 | M | AMMoL | IgG/λ | ND | 6 months |
| Parker (1973)[6] | 79 | M | AMMoL | IgG | Normal | Few weeks |
| Tursz et al (1974)[7] | 77 | M | AML | IgA/κ | ND | ND |
| 74 | M | AML | IgG/λ | ND | Few weeks | |
| Rosner and Grünwald (1974)[8] | 79 | M | AMMoL | ND | ND | Died shortly after diagnosis |
| Cleary et al (1978)[9] | 64 | M | AMMoL | IgA/κ | Normal | 4–5 months |
| Thiagrajan (1979)[11] | 72 | M | AML | IgA/κ | Aneuploidy | 5 months |
| Kastanas et al (1979)[10] | 70 | M | ND | IgG | ND | ND |
| Vallantin et al (1980)[11] | 69 | M | AML | IgG/λ | ND | Few weeks |
| Annino et al (1980)[12] | 74 | M | AMMoL | IgG/κ | Hypodiploidy;t (8;13) | 2 months |
| Parapia et al (1982)[1] | 79 | M | AMMoL | IgG/λ | Normal | 5 weeks |
| Naparstek et al (1982)[13] | 68 | M | AMoL | IgG/κ | ND | 15 months |
| Raz and Polliack (1984)[14] | 68 | M | AMoL | IgG/κ | Normal | 2.5 years |
| Kumar et al (2016)[15] | 73 | M | AML | IgG | ND | 4 months |
| Dash et al (1991)[16] | 32 | M | AML | IgG/λ | ND | ND |
| Thomas et al (2012)[17] | 73 | M | AMoL | IgA/κ | ND | 12 months |
| Thomas et al (2012)[17] | 70 | M | AML | IgG/κ | ND | 4 months |
| Anderson et al (1999)[18] | 51 | M | AML | κ | ND | 2 months |
| Luca and Almanaseer (2003)[19] | 77 | M | AMoL | IgG/λ | Monosomy 13; deletion of 20q | 1 month |
| Yanagimoto et al (2003)[20] | 82 | F | AML | λ | ND | ND |
| Attili et al (2006)[5] | 57 | F | AML | IgG/κ | 46 XY;t(8;21) | ND |
| Shukla (2008)[21] | 58 | F | AML | IgG/λ | ND | 1 month |
| Kim et al (2010)[22] | 51 | M | AML | κ | Complex | 11 months |
| Murukutla et al (2014)[23] | 66 | F | AML-M5 | κ | ND | 4 weeks |
| Lu-Qun et al (2015)[24] | 73 | M | AML | IgA/λ | ND | 6 months |
| Sashida et al (2003)[26] | 55 | F | AML-M1 | IgG/κ | ND | ND |
| Mailankody et al (2011)[27] | 73 | F | AML post MDS 5q- | IgG | ND | 12 months |
| Present case (2021) | 68 | M | AML | M spike -beta-2 globulin with kappa restriction | ND | Alive after 2 months of start of treatment |
Abbreviations: AML, acute myeloid leukemia; AML-M1, acute myeloid leukemia without maturation; AMMoL, acute myelomonocytic leukemia; AMoL, acute monocytic leukemia; FAB, French-American-British; IgA, immunoglobulin A; IgG, immunoglobulin G; ND, not defined.
Herein, we reported a case of simultaneous occurrence of AML and MM in a patient without previous exposure to chemotherapy. There is a possibility that these two malignancies may originate from common stem cells but it has not been supported with adequate evidence.
Malhotra et al reported 15 cases diagnosed with both Philadelphia chromosome-negative myeloproliferative neoplasms (MPNs) and MGUS or MM at their institute over a period of 5 years. Eleven patients with monoclonal gammopathy of undetermined significance (MGUS) and two patients with MM had received prior radiation treatment or chemotherapy and then developed MPNs. The two other patients with MM who did not receive any cytotoxic treatment developed myelofibrosis.[25]
Multiple gene mutations and deletions of RB-1, TP53, and IP32 and some susceptible genes may be involved in the simultaneous occurrence of both malignancies.[19,21] But the exact mechanism leading to the simultaneous occurrence of AML and MM is still debatable.
After technological advancements in diagnostic modalities and with introduction of newer principles of flowcytometry, fluorescence in situ hybridization (FISH), and karyotyping, genetic and molecular biomarkers have been discovered that play a key role in simultaneous occurrence of AML and MM.
Before 2003, all case reports of simultaneous presentation of AML and MM performed a type of serum immunofixation test that revealed the types of paraproteins in patients, including IgA, IgA/k, IgG, IgG/k, and IgG/λ; however, few cases conducted the chromosome type test, and the results displayed 46XY and hypodiploidy, as well as chromosomal abnormalities.[3,11]
Luca and Almanaseer in 2003 performed flowcytometry and demonstrated presence of myeloblasts with positive expression of Cd14, CD33, and HLA-DR, and a negative expression of CD45 for plasma cells with cytogenetics revealing Monosomy.[19–21] Kim et al reported a case of simultaneous presentation of AML and MM with k-type paraprotein with immunohistochemical test showing plasma cells to be positive for CD138 with kappa light chain restriction and myeloblasts to be positive for CD34 and CD117. Flow cytometry confirmed the presence of two distinct neoplastic populations of plasma cells and myeloblasts. FISH revealed a complex chromosomal pattern, with +5, +7, +8, +8q22, +11q23, −13q14, −16q22, +17q13.1, +20q12, and +21q22, and immunoglobulin heavy chain rearrangement.[22]
Prognosis remains extremely poor with no standard treatment established it because of its rare occurrence. Patients are often treated with therapy for AML since AML is more aggressive, and anthracycline group of drugs seems to be effective against MM as well.
Raz and Polliack described a 68-year-old man developing simultaneously with AML and MM and he was treated with a combination of vincristine, cyclophosphamide, melphalan, and prednisolone. This resulted in disappearance of monoblasts with decreased serum paraprotein level. Kim et al described a 51-year-old man who was concurrently diagnosed with AML and MM and treated with cytarabine and idarubicin along with bortezomib, followed by reinduction with mitoxantrone and high-dose cytarabine. But there was incomplete induction response and subsequently he underwent allogenic stem cell transplantation.[22]
Recently drugs like tipifarnib and bortezomib together have been shown to be synergistic in AML and MM cell lines, but usefulness of such therapies still needs a thorough evaluation.
Conclusion
Synchronous presence of AML with MM in a chemotherapy-naïve patient is an extremely rare occurrence. Concurrent diagnosis of these two hematological malignancies yields a poor prognosis and a proper treatment plan is still awaited.
Conflict of Interest
None declared.
Funding
None.
References
- Simultaneous presentation of acute myelomonocytic leukaemia and multiple myeloma. Acta Haematol. 1982;68(02):153-156.
- [CrossRef] [PubMed] [Google Scholar]
- Multiple myeloma and acute myelomonocytic leukemia: simultaneous occurrence without previous chemotherapy. Acta Haematol. 1980;64:195-200.
- [CrossRef] [PubMed] [Google Scholar]
- Plasmacytosis in acute myeloid leukemia: two cases of plasmacytosis and increased IL-6 production in the AML blast cells. Ann Hematol. 1998;76(06):273-277.
- [CrossRef] [PubMed] [Google Scholar]
- Concomitant myelomonocytic leukemia and multiple myeloma. Minn Med. 1972;55(05):446-448.
- [Google Scholar]
- Simultaneous occurrence of multiple myeloma and acute myeloid leukemia. Turk J Haematol. 2006;23(04):209-211.
- [Google Scholar]
- A case of acute myelomonocytic leukaemia associated with myelomatosis. Scand J Haematol. 1973;11(04):257-260.
- [CrossRef] [PubMed] [Google Scholar]
- Simultaneous occurrence of acute myeloblastic leukaemia and multiple myeloma without previous chemotherapy. Br Med J. 1974;2:642-643.
- [CrossRef] [PubMed] [Google Scholar]
- Multiple myeloma terminating in acute leukemia. Report of 12 cases and review of the literature. Am J Med. 1974;57(06):927-939.
- [CrossRef] [PubMed] [Google Scholar]
- Simultaneous presentation of acute myelomonocytic leukemia and multiple myeloma. Cancer. 1978;41(04):1381-1386.
- [CrossRef] [PubMed] [Google Scholar]
- Multiple myeloma and acute leukemia. Acta Haematol. 1979;62(02):78-80.
- [CrossRef] [PubMed] [Google Scholar]
- [Myeloma together with acute myeloblastic leukaemia in an untreated patient (author's transl)] Nouv Presse Med. 1980;9(35):2559-2560.
- [Google Scholar]
- Multiple myeloma and acute myelomonocytic leukemia: simultaneous occurrence without previous chemotherapy. Acta Haematol. 1980;64(04):195-200.
- [CrossRef] [PubMed] [Google Scholar]
- Simultaneous presentation of plasma cell and monocytic leukemia with a subacute clinical course. Acta Haematol. 1982;68(03):249-255.
- [CrossRef] [PubMed] [Google Scholar]
- Coexistence of myelomonocytic leukemia and monoclonal gammopathy or myeloma. Simultaneous presentation in three patients. Cancer. 1984;53(01):83-85.
- [CrossRef] [PubMed] [Google Scholar]
- Synchronous plasma cell myeloma and acute myeloid leukemia in a therapy-naïve patient: a rare occurrence. Indian J Hematol Blood Transfus. 2016;32(Suppl. 01):168-172.
- [CrossRef] [PubMed] [Google Scholar]
- An association of acute myeloid leukaemia and multiple myeloma: a case study. Indian J Cancer. 1991;28(01):45-47.
- [Google Scholar]
- Second malignancies after multiple myeloma: from 1960s to 2010s. Blood. 2012;119(12):2731-2737.
- [CrossRef] [PubMed] [Google Scholar]
- Primary myeloid leukemia presenting concomitantly with primary multiple myeloma: two cases and an update of the literature. Leuk Lymphoma. 1999;32(3-4):385-390.
- [CrossRef] [PubMed] [Google Scholar]
- Simultaneous presentation of multiple myeloma and acute monocytic leukemia. Arch Pathol Lab Med. 2003;127(11):1506-1508.
- [CrossRef] [PubMed] [Google Scholar]
- [Simultaneous occurrence of acute myeloid leukemia with multilineage dysplasia and multiple myeloma] Rinsho Ketsueki. 2003;44(01):19-24.
- [Google Scholar]
- Usha. Simultaneous appearance of dual malignancies of hematopoietic system-multiple myeloma and acute myeloid leukemia. Indian J Pathol Microbiol. 2008;51(01):118-120.
- [CrossRef] [PubMed] [Google Scholar]
- Simultaneous acute myeloid leukemia and multiple myeloma successfully treated with allogeneic stem cell transplantation. South Med J. 2010;103(12):1246-1249.
- [CrossRef] [PubMed] [Google Scholar]
- Concurrent acute monoblastic leukemia and multiple myeloma in a 66-year-old chemotherapy-naive woman. World J Oncol. 2014;5(02):68-71.
- [CrossRef] [PubMed] [Google Scholar]
- A case of simultaneous occurrence of acute myeloid leukemia and multiple myeloma. BMC Cancer. 2015;15:724-726.
- [CrossRef] [PubMed] [Google Scholar]
- Coexistence of myeloproliferative neoplasm and plasma-cell dyscrasia. Clin Lymphoma Myeloma Leuk. 2014;14(01):31-36.
- [CrossRef] [PubMed] [Google Scholar]
- Multiple myeloma with monosomy 13 developed in trisomy 13 acute myelocytic leukemia: numerical chromosome abnormality during chromosomal segregation process. Cancer Genet Cytogenet. 2003;141(02):154-156.
- [CrossRef] [PubMed] [Google Scholar]
- Risk of acute myeloid leukemia and myelodysplastic syndromes after multiple myeloma and its precursor disease (MGUS) Blood. 2011;118(15):4086-4092.
- [CrossRef] [PubMed] [Google Scholar]






