Translate this page into:
Unusual Immunophenotypic Heterogeneity in B-Lineage ALL
Address for correspondence: Dr. Prashant Sharma, Email: sharma.prashant@pgimer.edu.in
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
A three-year-old boy presented with fever, progressive pallor and aching limbs for the last 3 months followed by a petechial rash over the last 20 days. On examination, severe pallor, bony tenderness, petechiae over bilateral lower limbs and hepato-splenomegaly 8 cm and 4 cm below respective costal margins were noted. His haemoglobin was 34 g/L, total leukocyte count 69.3 × 10 9/L and platelet count was 14 × 109/L. A blood film showed 50% blasts and a bone marrow was performed, which revealed 93% blasts of lymphoid morphology that were myeloperoxidase-negative and PAS block-positive.
Flow cytometric immunophenotyping of the bone marrow, intriguingly, revealed two distinct blast clusters on the CD45-side scatter plot [Figure 1]. The numerically smaller one (~22% of all events, P1, red in figure) with dim intensity CD45 showed a typical precursor B-lymphoblastic immunophenotype:[1] positive for CD19, CD10, CD79a, CD34 and TdT along with CD38 and HLA-DR. The other cluster comprised ~67% of all events and was CD45 negative. It showed an immunophenotype more akin to progenitor B-lymphoblasts:[12] positive for CD19, CD79a, TdT, HLA-DR and CD38 but heterogeneous (dim to negative) for CD10 and negative for CD34. Both populations were negative for all T-lineage markers (surface and cytoplasmic CD3 and surface CD4, CD7 and CD8) as well as for all myeloid and monocytic markers (cytoplasmic myeloperoxidase and surface CD13, CD14, CD15, CD16, CD33, CD64, CD117 and CD123) tested.
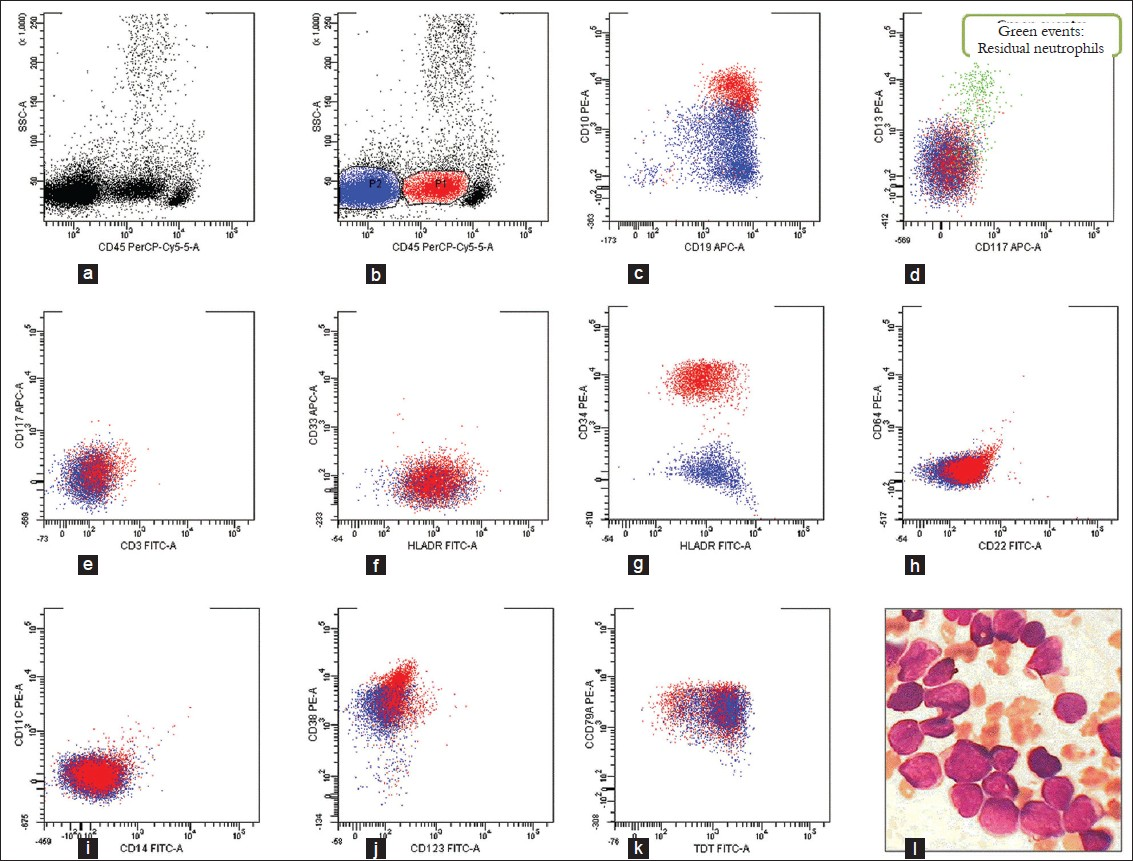
- Flow cytometric analysis of the bone marrow revealed 2 blast clusters on the CD45-side scatter plot that were gated separately (a, b) Events expressing dim intensity CD45 (P1, red) were distinct from another CD45-negative cluster (P2, blue) (c) Events in P1 cluster co-expressed CD19 and CD10 while P2 was CD19-positive with heterogeneous (negative to dim) CD10 (d, e, f) Both P1 and P2 were negative for CD13, CD117, CD3 and CD33 and positive for HLA-DR (g) P1 expressed CD34, while P2 was negative (h, i, j, k) Both P1 and P2 were negative for CD22, CD64, CD14, CD11c and CD123, and both population expressed CD38, CD79a and TdT (l) The bone marrow aspirate smears showed 93% small morphologically non-descript lympho-blasts. (MGG, ×1000)
A final diagnosis of B-cell acute lymphoblastic leukaemia (ALL) with mixed precursor and progenitor B-cell type blast populations was made. Molecular tests for t (9;22), t (4;11) and t (12;21) were negative. The boy was started on induction chemotherapy as per the unit's protocol and a bone marrow done at 28 days was in complete morphological remission.
The 2008 WHO classification of lymphoid neoplasms recognizes several categories of acute leukaemia of ambiguous lineage with flow cytometry forming the mainstay of their identification and typing.[3] However, intra-lineage heterogeneity (i.e. multiple populations confined to either B- or T- or granulocytic or monocytic lineages) is usually not considered to be of great diagnostic significance, although they may be seen, for instance, in acute myelomonocytic leukaemia with inv (16) abnormality. The older EGIL immunophenotypic classification of ALL still carries prognostic value, therefore it is intriguing to speculate whether our case actually has a true composite neoplasm: a standard risk pre-B-CALLA-positive ALL and a poorer risk CALLA-negative pro-B-ALL.[1–4] From a converse view, immunologically distinct immature and differentiated neoplastic population have long been described in childhood ALL [5] and perhaps our case is simply a dramatic illustration of this fact.
In either case, these unusual immunophenotypic findings reiterate the fact that even the extremely common B-ALL may occasionally spring a surprise for the flow cytometrist. It also presents a case for the reporting haematopathologist to maintain familiarity for the now less-and-less commonly employed EGIL classification of B-cell ALL.
REFERENCES
- Proposals for the immunological classification of acute leukaemia's.European Group for the Immunological Characterization of Leukaemia's (EGIL) Leukaemia. 1995;9:1783-6.
- [Google Scholar]
- Mixed lineage leukaemia-rearranged childhood pro-B and CD10-negative pre-B acute lymphoblastic leukaemia constitute a distinct clinical entity. Clin Cancer Res. 2006;12:2988-94.
- [Google Scholar]
- Acute leukaemia's of ambiguous lineage. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, eds. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: WHO Press; 2008. p. :150-5.
- [Google Scholar]
- Flow cytometry immunophenotyping of hematolymphoid neoplasia. Methods Mol Biol. 2011;699:295-316.
- [Google Scholar]
- Immature and differentiated neoplastic populations in acute lymphoid leukemia of childhood: Biological and clinical implications. Leuk Lymphoma. 1993;11:1-7.
- [Google Scholar]




