Translate this page into:
Use of culture- and ELISA-based toxin assay for detecting Clostridium Difficile, a neglected pathogen: A single-center study from a tertiary care setting
Address for correspondence: Dr. Sujata Lall, 15-L Model Town, Rohtak - 124 001, Haryana, India. E-mail: sujatamed@gmail.com
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
INTRODUCTION:
Clostridium difficile is a Gram-positive spore-bearing anaerobic bacillus increasingly associated with both community- and hospital-acquired colitis and diarrhea. It is the most common identifiable bacterial cause of healthcare-associated diarrhea associated with antibiotic use and one of the most common anaerobic infections. The diagnosis of C. difficile infection includes detection of toxin A/B in stool specimens by direct enzyme immunoassay, culture of pathogen from the stool specimens using a selective agar Cycloserine-Cefoxitin fructose agar (CCFA), tissue culture assay, and detection of glutamate dehydrogenase an enzyme produced by C. difficile. With few reports from India on this disease, the present study was planned to throw more light on the prevalence and utility of laboratory diagnostic methods for C. difficile-associated diarrhea (CDAD).
MATERIAL AND METHODS:
After taking approval from the Ethics Committee, 150 patients with antibiotic-associated diarrhea were taken as a study group and fifty patients with exposure to antibiotics but who did not develop diarrhea were taken as controls. Stool specimen was processed for both culture on CCFA and toxin detection by IVD Tox A + B ELISA.
RESULTS:
Only four specimens were culture positive, whereas 13 were ELISA positive. All culture-positive isolates were toxigenic. C. difficile was neither isolated nor its toxin detected in the control group. Culture- and toxin-based assays may not detect all cases of CDAD.
CONCLUSION:
Based on the results of the present study, culture does not provide any additional yield over toxin assay. Better diagnostic modalities would be required to prove CDAD.
Keywords
Clostridium difficile
culture
ELISA
Introduction
Clostridium difficile is a Gram-positive spore-bearing anaerobic bacillus increasingly associated with both community- and hospital-acquired colitis and diarrhea. It is the most common identifiable bacterial cause of nosocomial diarrhea associated with antibiotic use and one of the most common anaerobic infections.[1] C. difficile-associated diarrhea (CDAD) is a life-threatening disease with an attributable mortality of 6%–15% and up to 25% in frail elderly people.[2] The clinical presentations in increasing order of severity include asymptomatic carriage, colitis without pseudomembrane formation, pseudomembranous colitis (PMC), and fulminant colitis.[3] Damage to the normal colonic flora serves as a prerequisite for infection by the bacterium.[4] The incidence of CDAD is on the rise and a newer ribotype, NAP1/BI/027, has been found to be responsible for multiple recent outbreaks in various parts of the world.[56]
The diagnosis of C. difficile infection includes the detection of toxin A/B in stool specimens by direct enzyme immunoassay (EIA), culture of pathogen from the stool specimens using a selective agar cycloserine-cefoxitin fructose agar (CCFA), tissue culture assay to demonstrate the cytopathic effect of toxin on various cell lines which is seen as cell rounding, detection of glutamate dehydrogenase (GDH) an enzyme produced by C. difficile, and nucleic acid amplification tests for the toxin. Among these, tissue culture assay is considered as the gold standard.[7] However, detection of toxin using ELISA is preferred by diagnostic laboratories because of its ease. ELISA-based cytotoxin assays are reported to have sensitivity and specificity ranging from 50%–90% to 70%–95%, respectively.[8]
Considering the changing epidemiology of CDAD, it is important to diagnose it as early as possible both to prevent transmission as well as for better patient management. With few reports from India on this disease,[910111213] the present study was planned to throw more light on the prevalence and utility of laboratory diagnostic methods for CDAD.
Materials and Methods
After obtaining permission from the Ethics Committee (No-EC/154/2011), a prospective case–control study was carried out, from January 2012 to December 2013 in a tertiary care hospital. Assuming that C. difficile infection rate is 10% among the patients with antibiotic-associated diarrhea (AAD) and alpha error 5%, we needed to enroll 144 cases. Further assuming that 5% of the samples were contaminated, so it was decided to enroll 150 cases along with 50 controls. We could not add more controls due to financial constraints for the study. Medicine and allied departments and pediatric departments were requested to send stool samples from patients who satisfy inclusion criteria after taking written informed consent of study patients or their parents/guardians and controls. Furthermore, the investigator visited different wards to identify patients fitting inclusion criteria. All patients from medical and pediatric wards of any age/gender were included who satisfied the following inclusion criteria: (1) history of antibiotic use either in the previous month or recently since 5 days and who developed diarrhea and (2) patients with PMC detected on lower gastrointestinal endoscopy referred for C. difficile detection and no other recognized etiology of diarrhea.[14] Controls were those patients admitted during the study period who had taken antimicrobials for at least 5 days but did not develop diarrhea. Diarrhea was defined as six watery stools over 36 h or three unformed stools in 24 h for 2 days or eight unformed stools over 48 h.[15] Patients who had diarrhea during the first 72 h of admission in a hospital, neonates, and psychiatric patients were excluded from the study. A detailed study pro forma was filled up for each one of them, which included age, gender, severity of diarrhea[16] with duration, association with other symptoms such as abdominal pain, fever, any other illness, ward and unit of admission, antibiotic history, other significant laboratory investigations, duration of hospital stay, and provisional diagnosis.
Microbiological method
Specimen collection
Fecal samples were collected from AAD cases in sterile, wide-mouth, screw capped containers and immediately transferred to the laboratory, preferably within 2 h. Specimens were processed for microscopy, anaerobic culture, and ELISA. For ELISA, freshly collected specimens were kept at 2–8°C and tested within 24 h of collection. Specimens that could not be tested within this time were frozen at −20°C or lower until used. Freezing does not adversely affect the test as per the literature of ELISA kit used. The gross description of the stool specimen was recorded.
Microscopy
A direct wet mount for fecal leukocytes and a Gram's stain for detecting organisms with characteristic morphology as that of C. difficile which appear as a Gram-positive bacillus with subterminal spore were carried out.
Culture
For C. difficile isolation, stool samples were inoculated into Robertson's cooked meat (RCM) broth for enrichment and incubated anaerobically at 37°C for 24–48 h. Samples were also directly plated on CCFA. RCM was subcultured after 48 h on CCFA. All the plates were incubated anaerobically in McIntosh Fildes jar for 48–72 h. Anaerobiosis was monitored as per standard protocol by keeping a known strain of Pseudomonas aeruginosa inoculated in a citrate slant in the jar. Appropriateness of the prepared medium was checked for sterility as well as its property to support growth of C. difficile. Validation of the method of isolation of C. difficile by culture was done by subculture of a known standard strain of C. difficile (ATCC 9689) on CCFA, procured from HiMedia in KWIK–STIK form, and incubated anaerobically. These EZ-Accu Shot Microorganisms are lyophilized microorganism preparations that provide challenges of <100 colony-forming unit/0.1 mL and are recommended for the growth promotion quality control of culture media. The media were considered valid if characteristic growth was observed.
Plates were examined initially no later than after 48 h of incubation for optimal selectivity. After 48 h of incubation on CCFA, colonies of C. difficile were 4 mm or larger, flat to slightly raised rhizoid which had a speckled opalescence and strong horse manure-like odor. If the colonies resembling C. difficile were not detected, the plates were reincubated for a further 48 h and visually screened for C. difficile before being discarded. Colonies of distinctive morphology were stained with Gram's stain and subcultured in RCM medium. After 3 days of incubation, the cultures were checked for purity. A test for aerotolerance was done to confirm that the isolate was an obligate anaerobe. Positive cultures were identified by gross colonial morphology, Gram's stain characteristics, and standard biochemical tests. Glucose, fructose, and mannose were fermented, and lactose and sucrose were not fermented. Gelatin was liquefied and lecithinase was not produced.
Toxin assay ELISA
For toxin assay, C. difficile toxin A + B Stool Antigen Microwell ELISA Kit manufactured by IVD Research Inc., Carlsbad, USA, was used. This ELISA is an in vitro immunoassay for the qualitative determination of C. difficile toxins A + B in feces. It is a double antibody (sandwich) ELISA using anti-toxin A + B antibodies to capture the antigen from the stool supernatant. A second set of anti-toxin A + B antibodies is added which sandwiches the captured antigen. This reaction is visualized by the addition of an anti-second antibody conjugated to peroxidase and chromogen tetramethylbenzidine. The resulting yellow color development indicates the presence of C. difficile toxins A + B being bound by the antibodies. The test was carried out as per manufacturer's instructions.
Any sample well that was obviously more yellow than the negative control well or gave an absorbance reading of 0.15 optical density (OD) units and above indicated that the sample contained C. difficile toxin. Similarly, any sample well that was not obviously more yellow than the negative control well or gave an absorbance reading <0.15 OD units indicated that the sample did not contain detectable levels of C. difficile toxin. All wells were read at 450/620–650 nm.
Patients whose stool samples gave a positive reaction in ELISA and/or which grew C. difficile on culture were considered as cases and referred to as patients with CDAD, and other patients were referred as simply patients with AAD. All the patients were followed up for their response to discontinuation of antibiotic therapy and/or treatment with metronidazole or vancomycin.
Statistical analysis
The study participants were divided into two groups: Group A was those with AAD in whose stool specimen C. difficile or its toxin was detected and Group B was those with AAD in whose stool specimen C. difficile was not detected. Data were analyzed by frequency percentage. To determine the significance of the value obtained, Chi-square test was used, P </= 0.05 was considered statistically significant.
Results
One hundred and fifty patients who gave a history suggestive of antibiotic-associated diarrhea were included in the study. Of these, 31 were children (<12 years and minimum age 4 years) and 119 were adults. Fifty age- and gender (for adult group)-matched controls were also taken from the same hospital setting. Out of 150 patients in the study group, C. difficile was isolated from the stool of four patients (Three adults and one child). Thirteen fecal samples tested positive for toxin A + B by EIA (11 adults and two children). All the four samples tested positive by culture were found to carry C. difficile toxin by ELISA. No fecal sample from the control group was positive for C. difficile either by culture or ELISA. No additional yield was obtained by enrichment with RCM. No additional yield was obtained by reincubation after 48 h.
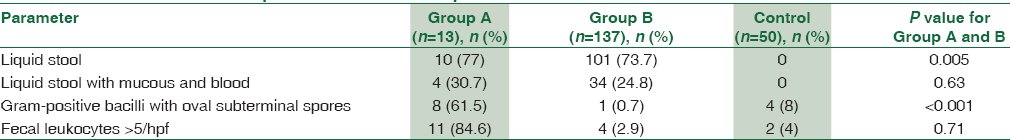
Main presenting features of both Groups A and B are listed in Table 1.

Age and gender distribution in the two study groups and controls was analyzed. Maximum cases were from 31 to 45 years age group, males (nine) were more than females (four).
Evaluation of stool samples for routine parameters was done as shown in Table 2.
Of 13 positive cases of C. difficile, two patients died. Mortality was attributed to chronic renal failure in one patient and septicemia in another. Seven patients responded on stopping the initial antibiotic which included a third-generation cephalosporin and/or an aminoglycosides or clindamycin. Two patients responded successfully to treatment with oral metronidazole and two more patients required an additional oral vancomycin therapy.
Discussion
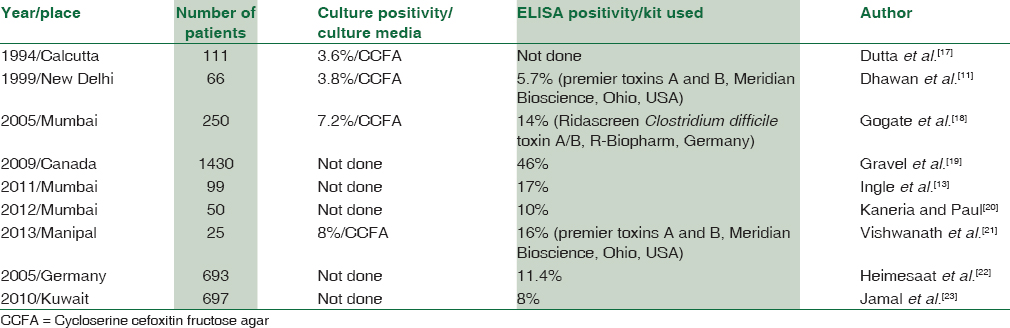
The present study on 150 patients with AAD and 50 controls was carried out to determine the prevalence of CDAD using culture and toxin assay. In the present study, 8.67% of suspected AAD cases were either culture positive or toxin assay positive for C. difficile. The culture positivity rate was 3.34%. Low culture positivity rates have been documented in other studies as given in Table 3. The higher rates in some of the studies may be attributed to a low sample size[21] or due to the bias of results with a study done on children of age group of 5–12 years as participants.[18] Children are reported to have higher colonization rates of C. difficile.[1]
Lower culture positivity rates can be due to delay in sample transportation to the laboratory, inefficient management of anaerobiosis due to repeated subculture of the isolate which leads to loss of viability, C. difficile being overgrown by many other microorganisms on CCFA, and dilutional effects of diarrhea as culture is dependent on the presence of spores or viable vegetative cells.[24] Culturing of nondiarrheal stools also leads to false-negative results.[25]
In the present study, the specimens were transported and processed within an hour of collection. Anaerobiosis was maintained during incubation of the specimens according to the established standards, repeated subculture of the isolates was avoided, and all the stool specimens were diarrheic. Hence, the lower isolation rate in the present study may be due to sampling error inherent to uneven distribution of C. difficile in the fecal samples or other unexplained reasons. Only one stool specimen per patient was processed. Inclusion of multiple stool specimens may have increased the yield but was not attempted due to cost constraints.
Currently, stool culture is the method least employed by hospitals owing to limitations of cost and turnaround time of approximately 48 h. No additional yield was obtained after incubating for further 48 h or by enriching it on RCM media. The turnaround time in the present study was also 3 days. In addition, the accuracy of this method varies considerably in different laboratories because the methods and culture media are not standardized. The culture media used in the present study were CCFA. Various other media and their recovery rates, which have been evaluated for the isolation of C. difficile include (Chrome–ID C. difficile agar 98%), CLO (C. difficile selective agar 97%), BBL (B. D. chrome agar for C. difficile, 97%), (Cefsulodin Cycloserine Egg Yolk [CCEY] Agar, 98%), Oxoid C. difficile selective agar (98%), and CCEY/L (CCEY Agar with lysozyme 96%.[26] CCFA has been used in the present study because it is effective, economical, and readily available selective media.
Infectious Disease Society of America (IDSA) currently recommends that a positive stool culture followed by identification of a toxigenic isolate, as performed by an experienced laboratory, provides the standard against which other clinical test results should be compared (SHEA IDSA).[14] In the present study, 8.67% of stool samples were positive for C. difficile toxin A + B. All the cultured isolates were toxigenic. The ELISA positivity rates range from 10% to 17% in various Indian studies[2728] and up to 30%[29] in Western literatures. ELISA has a good specificity; however, 100–1000 pg of both toxin A and toxin B must be present for the test to be positive.[27] The kit used in the present study has the capacity to detect approximately 2 ng/ml of toxin A and 3 ng/ml of toxin B. Hence, a false-negative rate of 10%–20% may occur.[25] A false-negative result can also be caused by proteolytic decomposition of the toxins due to inappropriate storage of the sample.[14] In the present study, as the ELISAs were run in batches, the stool specimens were stored at −20°C immediately after culturing, and hence the possibility of toxin decomposition is negligible. Toxin assay has the advantage of lower turnaround time and ease of performance; however, the high cost per single test may necessitate batching of samples due to usage of other kit components such as sample diluent, wash buffer, and controls. The cost per test for toxin assay in the present study was approximately Rs 200/. This does not include the cost of equipment and workforce but only that of the consumables. As per the kit literature, the assay has a limitation of not giving accurate results on a concentrated sample. In the present study, samples were not concentrated. Furthermore, multiple samples over time are indicated to improve yield, but this could not be achieved due to difficulty in follow-up and cost constraints.
Prevalence of CDAD is around 2%–4% in patients without diarrhea and 7%–30% in patients with diarrhea in different hospital-based studies.[17182127] In the present study, the prevalence of C. difficile was 8.67% in hospitalized diarrhea patients and 0% in nondiarrhea controls. All the cases and the controls were on antibiotics. Gupta and Yadav[30] have reported C. difficile isolation rate of 25.3% in hospitalized patients with diarrhea and 4.3% in controls admitted for other ailments. Niyogi et al.[10] have reported 4% in hospitalized patients with diarrhea and 2.7% in nondiarrhea controls. Bhattacharya et al.[27] isolated C. difficile as a sole pathogen from 7.3% of 233 patients with acute diarrhea. Vaishnavi et al.[28] reported 30% positivity for C. difficile toxin in hospitalized patients of all age groups receiving single to multiple antibiotics for various diseases, but only in 7% of patients not receiving antibiotics. Some recent studies estimated a prevalence rate of 10%,[20] 14%,[18] and 17%.[13] The isolation of C. difficile in nondiarrhea controls in other studies may be related to colonization. Colonization by C. difficile in asymptomatic adults depends on the presence of long-standing disease, contact with suspected patient of CDAD, and length of hospital stay which increases the chances of contact with spores.[1] Low carriage rates in asymptomatic adults in the present study may be due to very low number of CDAD patients, thereby minimizing exposure risk, inclusion of nondiarrheal controls, and incorporation of all age groups rather than only pediatric population which shows high carriage rate.
Among patients positive for C. difficile, fever, abdominal pain, and diarrhea have been reported to be more common.[31] In the present study, abdominal pain and abdominal distension were significant predictors of the disease (P = 0.03), whereas severe diarrhea and fever were not. Thompson et al.[29] had reported fever, abdominal pain, and distension as the predominant symptoms. Similar results have been documented in other studies.[1718] Liquid stool (P = 0.005) and Gram-positive bacilli with oval subterminal spores (P < 0.001) were also considered as significant predictors of disease.
CDAD has been reported to be more common in women and older patients.[12] Studies from India have reported varying male-female ratios. In the present study, among 13 positive cases, nine were males (60.9%) and four were females. A maximum number of positive cases were found in the age group of 30–45 years followed by those more than 45 years of age. Similar male preponderance and higher age association have been reported in other studies from India.[122120] The increased risk of acquiring C. difficile infection in the elderly may be due to age-related changes in fecal flora, immune senescence, impaired ability of neutrophils to phagocytose and kill C. difficile and decrease in the capacity of serum to neutralize toxins with increasing age, or the presence of other underlying diseases.
The present study had certain limitations. Due to cost constraints, the sample size of the controls was restricted to 50. Due to the absence of facilities for tissue culture, cytotoxin assay for C. difficile which is considered the gold standard could not be carried out.
Since other reports highlight the increasing prevalence of C. difficile as the causative agent of AAD, it is important to lay some protocol for correct isolation of the organism in cases of AAD. To increase the yield of laboratory detection, one potential strategy recommended by IDSA is a two-step method that uses EIA detection of GDH as initial screening test followed by the cell cytotoxicity assay or toxigenic culture as the confirmatory test for GDH-positive stool specimens only. As reported in literature, antibiotic-associated C. difficile diarrhea is posing a new challenge to health-care settings with its changing epidemiology and emerging hypervirulent strain NAP1. Unfortunately, it is still a neglected diagnosis due to tedious anaerobic methods, costly serological techniques, and nonavailability of tissue culture laboratory.
Conclusion
Culture- and toxin based assays may not detect all cases of CDAD. Based on the results of the present study, culture does not provide any additional yield over toxin assay. Better diagnostic modalities would be required to prove CDAD.
Financial support and sponsorship
This study was supported by Department of Microbiology Seth GSMC and KEMH Mumbai and Diamond Jubilee Society Trust, Seth GSMC and KEMH Mumbai.
Conflicts of interest
There are no conflicts of interest.
References
- Established and potential risk factors for Clostridium difficile infection. Indian J Med Microbiol. 2009;27:289-300.
- [Google Scholar]
- Clostridium difficile infection: Clinical spectrum and approach to management. Indian J Gastroenterol. 2011;30:245-54.
- [Google Scholar]
- Clinical spectrum and pathogenesis of Clostridium difficile associated diseases. Indian J Med Res. 2010;131:487-99.
- [Google Scholar]
- Spread and epidemiology of Clostridium difficile polymerase chain reaction ribotype 027/toxinotype III in the Netherlands. Clin Infect Dis. 2007;45:695-703.
- [Google Scholar]
- Clostridium difficile colitis: An increasing hospital-acquired illness. Am J Surg. 1995;169:480-3.
- [Google Scholar]
- Laboratory diagnosis of Clostridium difficile-associated diarrhoea: A plea for culture. J Med Microbiol. 2005;54(Pt 2):187-91.
- [Google Scholar]
- Diagnostic approach to Clostridium difficile infection. Indian J Gastroenterol. 2010;29:137-9.
- [Google Scholar]
- Prevalence of Clostridium difficile in pseudomembranous and antibiotic-associated colitis in North India. J Diarrhoeal Dis Res. 1986;4:157-60.
- [Google Scholar]
- Prevalence of Clostridium difficile in hospitalised patients with acute diarrhoea in Calcutta. J Diarrhoeal Dis Res. 1991;9:16-9.
- [Google Scholar]
- Incidence of Clostridium difficile infection: A prospective study in an Indian hospital. J Hosp Infect. 1999;43:275-80.
- [Google Scholar]
- Changing pattern of Clostridium difficile associated diarrhoea in a tertiary care hospital: A 5 year retrospective study. Indian J Med Res. 2008;127:377-82.
- [Google Scholar]
- Prevalence and clinical course of Clostridium difficile infection in a tertiary-care hospital: A retrospective analysis. Indian J Gastroenterol. 2011;30:89-93.
- [Google Scholar]
- Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431-55.
- [Google Scholar]
- Clostridium difficile-associated diarrhea and colitis. Infect Control Hosp Epidemiol. 1995;16:459-77.
- [Google Scholar]
- A prospective multicentric observational study to evolve the usefulness of the predefined homoeopathic medicines in the management of acute diarrheal disease in children. Indian J Res Homoeopathy. 2009;3:21-8.
- [Google Scholar]
- Clostridium difficile in antibiotic associated paediatric diarrhea. Indian Pediatr. 1994;31:121-6.
- [Google Scholar]
- Diagnostic role of stool culture and toxin detection in antibiotic associated diarrhoea due to Clostridium difficile in children. Indian J Med Res. 2005;122:518-24.
- [Google Scholar]
- Health care-associated Clostridium difficile infection in adults admitted to acute care hospitals in Canada: A Canadian Nosocomial Infection Surveillance Program Study. Clin Infect Dis. 2009;48:568-76.
- [Google Scholar]
- Incidence of Clostridium difficile associated diarrhoea in a tertiary care hospital. J Assoc Physicians India. 2012;60:26-8.
- [Google Scholar]
- Clostridium difficile infection at a tertiary care hospital in South India. J Assoc Physicians India. 2013;61:804-6.
- [Google Scholar]
- Prevalence of Clostridium difficile toxins A and B and Clostridium perfringens enterotoxin A in stool samples of patients with antibiotic-associated diarrhea. Infection. 2005;33:340-4.
- [Google Scholar]
- Analysis of prevalence, risk factors and molecular epidemiology of Clostridium difficile infection in Kuwait over a 3-year period. Anaerobe. 2010;16:560-5.
- [Google Scholar]
- Selective and differential medium for isolation of Clostridium difficile. J Clin Microbiol. 1979;9:214-9.
- [Google Scholar]
- Clostridium difficile-associated diarrhea: A review. Indian Med Gaz. 2011;145:481-94.
- [Google Scholar]
- Evaluation of a chromogenic culture medium for isolation of Clostridium difficile within 24 hours. J Clin Microbiol. 2010;48:3852-8.
- [Google Scholar]
- Clinical manifestation of Clostridium difficile enteritis in Calcutta. J Assoc Physicians India. 1991;39:683-4.
- [Google Scholar]
- Detection of Clostridium difficile toxin by an indigenously developed latex agglutination assay. Trop Gastroenterol. 1999;20:33-5.
- [Google Scholar]
- Clostridium difficile cytotoxin in a pediatric population. Am J Dis Child. 1983;137:271-4.
- [Google Scholar]





