Translate this page into:
Vaginal cytomorphological profile in correlating suicidal deaths in medicolegal autopsy cases
*Corresponding author: Jayanthi Yadav, Department of Forensic Medicine and Toxicology, All India Institute of Medical Sciences, Bhopal, Madhya Pradesh, India. jayanthi.fmt@aiimsbhopal.edu.in
-
Received: ,
Accepted: ,
How to cite this article: Sangita M, Yadav J, Chandela RK, Arora A. Vaginal cytomorphological profile in correlating suicidal deaths in medicolegal autopsy cases. J Lab Physicians. 2024;16:393-8. doi: 10.25259/JLP_85_2024
Abstract
Objectives:
Vaginal cytology is a sensitive method for assessing women’s hormonal status, showing variations in menstrual cycle phases. Studies suggest a higher rate of female suicides during the luteal phase. This study aimed to link vaginal cytomorphological profiles with the manner of death and age of the deceased.
Materials and Methods:
Vaginal smears from 64 autopsy cases were microscopically examined for maturation index (MI) and value, reflecting hormonal profiles.
Statistical Analysis:
ANOVA between the mean of maturation value with manner of death was found to be significant.
Results:
Among the 48 suicides, 54.2% showed predominantly intermediate cells in the luteal phase, indicating a mid-zone shift in the MI. The remaining 16 non-suicidal cases showed fewer intermediate cells. Suicides shared a similar cytological profile and MI, especially in the reproductive age group.
Conclusions:
While vaginal smears are typically used in sexual assault cases to detect sperm, they also offer insights into women’s hormonal profiles. Understanding these profiles could provide clues about psychological status and its relation to the manner of death beyond sexual assault.
Keywords
Vagina
Cytology
Suicide
Age group
Menstrual cycle
INTRODUCTION
In the field of Forensic Medicine, vaginal smears are mostly studied to diagnose sexual offenses. However, the study of vaginal cytology has vast applicability and has long been acknowledged as a supremely suited and extremely sensitive and simple tool for determining a woman’s hormonal condition.[1,2] According to the phases of their menstrual cycle, different age groups of females exhibit varied vaginal cytological pictures. Cytological interpretation of hormone effects is based on the thickness and maturation of the vaginal mucosa, which are dependent on the type and concentration of circulating sex hormones.[2-4] According to a number of academic studies, female suicides are more common among women with premenstrual syndrome or premenstrual dysphoric disorder (PMDD), which happens in the luteal phase of the menstrual cycle.[5-11]
The vaginal wall is lined by stratified squamous and are arranged in layers of cells of different morphology. There are three main layers of cells: (i) superficial, (ii) middle, and (iii) deep. The thicknesses of these different layers of cells depend on the hormonal status of the female, mainly estrogen, which, in turn, depends on the different phases of the menstrual cycle.[2] Superficial cells are further divided into cornified and pre-cornified cells, and both have pyknotic nuclei. Precornified cells are larger hexagonal or octagonal flat wafers such as cells and have faintly basophilic cytoplasm as compared to cornified cells. Cornified cells represent the final phase of complete estrogenic maturity. It has pink eosinophilic cytoplasm and the largest amount of cytoplasm of any vaginal cell. The superficial layer is the thickest, and vaginal smears are predominantly superficial cells in the second half of follicular phases when the estrogen level is high. The middle layer has intermediate cells, which are large ellipsoid polygonal with smoothly rounded edges and have vesicular nuclei. Cytoplasm stains more faintly, and the nucleus is smaller and less deep staining than in the cells of the deep layer. These cells will be seen predominantly in smears taken during the luteal phase.[1] The deep layer consists of two types of cells – (i) basal cells and (ii) parabasal cells. The basal cell is less mature, smaller, and more basophilic. They are round cells with basophilic cytoplasm and a relatively large central nucleus, which is uniform in shape and size. Vaginal smears where this cell predominates are typical of low estrogen content, for example, menopausal, lactating, or postpartum smears. Parabasal cells are similar to the basal but slightly more mature. The presence of parabasal cells in vaginal smear indicates a low estrogenic influence during menopause, lactating, and postpartum.[1,3] It is also characteristic of the desquamation of vaginal epithelium, which may result from vaginal infection or basal hyperplasia. A vaginal smear of the menstrual phase is characterized by immature cells, endometrial debris, and blood cells.
There are several ways of semi-quantitative assessment of hormones. Mention may be made of the karyopyknotic index, maturation index (MI), maturation value (MV), crowded cell index, and navicular cell index. In this study, we have assessed the MV and MI in order to evaluate the possible phase of the menstrual cycle and to correlate with the manner of death and age. MI is defined as a percentile relationship of the differential count of three main cells of the vaginal epithelium, that is, parabasal cells, intermediate cells, and superficial cells. It is determined by the differential cell count method and expressed in three-number series, which add to a hundred and are described as zones. The left number indicates the percentage of parabasal cells, the middle one indicates the percentage of intermediate cells, and the right one indicates the percentage of superficial cells. The largest of the three numbers indicates the zone shift of vaginal cytology. MI will show a left shift when parabasal cells are more, mid-zone shifts when intermediate cells are more, and a right shift when superficial cells are more.
MV is an integrative biomeasure of estrogenization of women, useful for analyses of health and sexuality.[12,13] It is calculated by multiplying with a fixed multiplication factor to the number of each type of cell indicated in MI. MV is calculated from the differential cell count using the formula “MV = 1.0 × Superficial cells + 0.5 × Intermediate cells +0.0 × Parabasal cells”.[1] Its value varies from 40% to 80% in the first half of the menstrual cycle and is highest when estrogen is at its peak level.[11] In forensic practice, the focus lies in determining the presence of sperm, seminal content, DNA profiling, and bacteriological culture in sexual assault culture.[14] The morphology of vaginal cells is rarely studied. In regards to infrequent study of vaginal study in autopsy cases, this study was done to see the correlation of vaginal cytology in cases with different manners of death.
MATERIALS AND METHODS
This study is an autopsy-based cross-sectional study done on 64 cases of different manners of death. This study was done with the aim of correlating the cytomorphological profile in relation to the manner of death and also to find if suicidal cases show the cytological profile of a particular phase of the menstrual cycle. The vaginal smear was collected during a medicolegal autopsy, with time since death varying from 6 h to 48 h. For sample collection, a sterile swab was taken and moistened with distilled water/saline water. The swab was then inserted in the posterior fornix and rotated 360°. The swab was dabbed and rotated on a clean slide to prepare the smear. The smear is then air-dried and dipped in absolute alcohol (95% ethanol) for 15 min. The slides were rinsed in water before dipping in hematoxylin stain for 5 min. The slides were rinsed in water and dipped in eosin stain for 2 min, followed by 2 dips in absolute alcohol. The slides were dried and examined under a microscope. We included slides with good cell distribution and good staining and excluded slides with fewer than 100 cells, faint staining, and cell fragments. A differential vaginal epithelial cell count was done by examining the slides in a longitudinal strip fashion and noting the observation on a 100-cell count table. MV was calculated from the differential cell count using the formula “MV = 1.0 × Superficial cells + 0.5 × Intermediate cells + 0.0 × Parabasal cells”. A number of superficial cells [Figure 1], intermediate cells [Figure 2], and parabasal cells [Figure 3] determine the left zone, mid zone, and right, respectively, of MI. Thus, the differential count will determine the zonal shift of MI. The data collected from the study were entered in the MS EXCEL spreadsheet. Analysis was carried out using the Statistical Package for the Social Sciences version 26. According to the manner of death, the cases were grouped into suicidal, accidental, natural, homicidal, and cases where the cause of death cannot be determined. The cases were divided into three age groups: 0–14 years, 15– 45 years, and above 45 years. The study was approved by the Institutional Human Ethics Committee vide letter no. IHECLOP/2023/IL095 dated April 13, 2023. As the vaginal slides were prepared as part of a medicolegal autopsy, consent from relatives was not taken, but the identity of the individual was protected as per ethical guidelines.
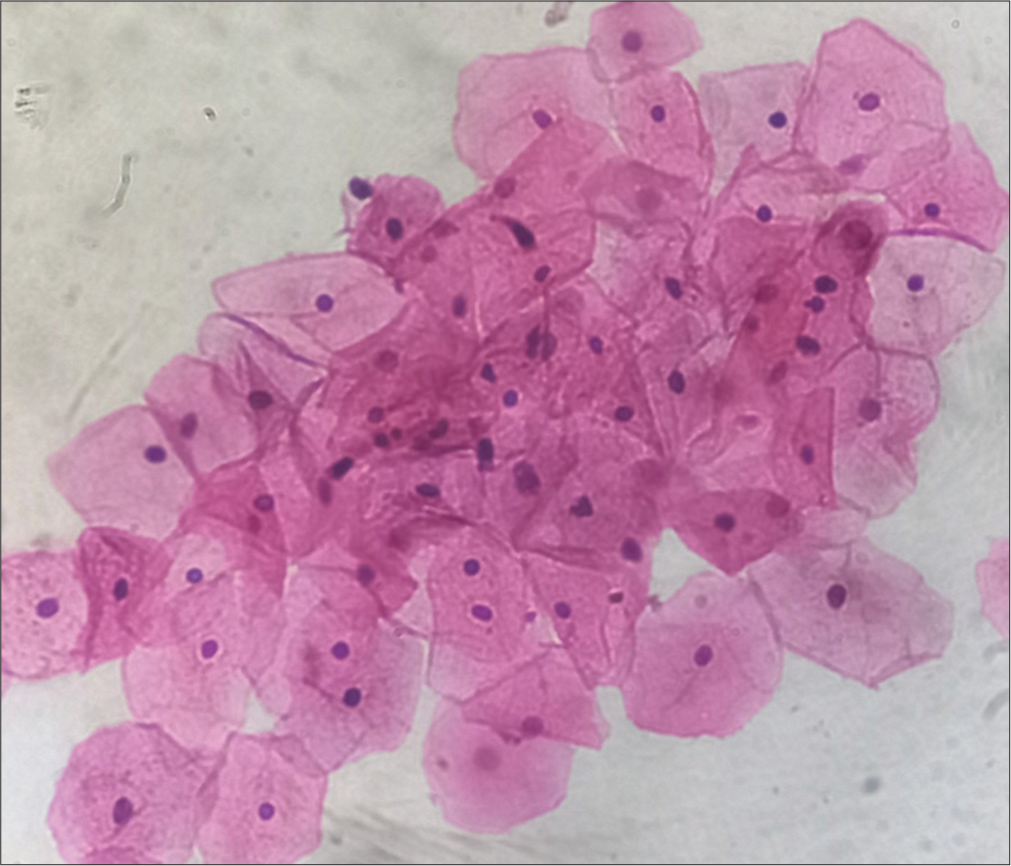
- Predominantly superficial cells, hematoxylin, and eosin (H&E) stain, ×40.
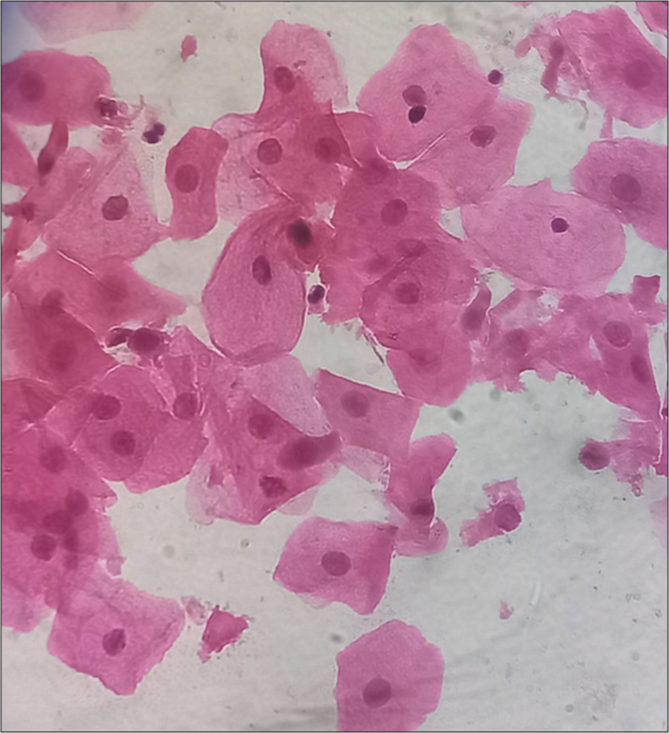
- Predominantly Intermediate cells, hematoxylin, and eosin (H&E) stain, 40x.
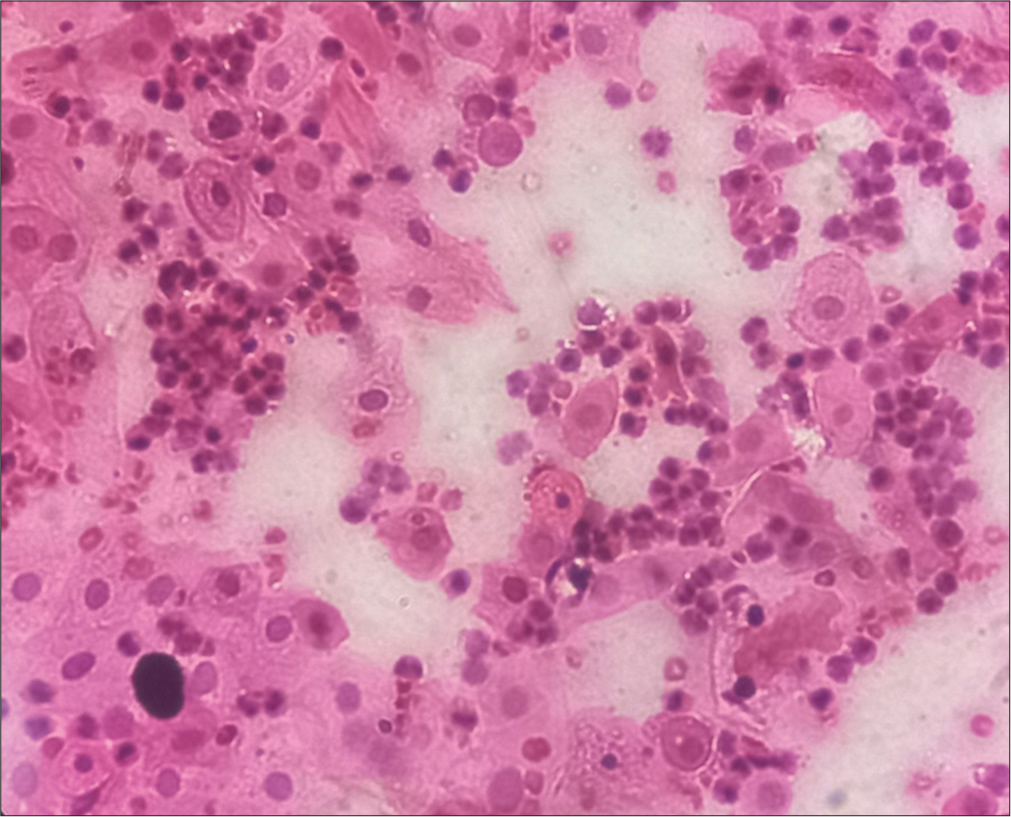
- Predominantly Parabasal cells, hematoxylin, and eosin (H&E) stain, 40x.
RESULTS
A total of 64 cases were studied in the age group of 13–75 years. The cases were collected within 6–48 h after death during medicolegal autopsy. The mean MV of total cases was 57.36, of which 33 cases had mid-zone shift, 21 had right zone shift, and 10 cases showed left zone shift of MI [Table 1].
| Case distribution | ||||
|---|---|---|---|---|
| Zone shift | Total | |||
| Left shift | Midzone shift | Right shift | ||
| Age | ||||
| 0–14 years | 0 | 1 | 2 | 3 |
| 15–45 years | 7 | 31 | 18 | 56 |
| Above 45 years | 3 | 1 | 1 | 5 |
| Total | 10 | 33 | 21 | 64 |
| Manner of death | ||||
| Accident | 3 | 6 | 1 | 10 |
| Cannot determine | 0 | 1 | 1 | 2 |
| Homicide | 0 | 0 | 1 | 1 |
| Natural | 2 | 0 | 1 | 3 |
| Suicide | 5 | 26 | 17 | 48 |
Corelation with age
Out of the total 64 cases, 56 cases (93.75%) cases were in the reproductive age group (15–45 years of age), five cases were above 45 years, and three cases were below 15 years. The youngest case was of age 13 years, and the oldest was 75 years old. Out of the 56 cases in the reproductive age group, 31 cases showed mid-zone shift of MI, 18 cases showed right zone shift, and seven cases showed left zone shift. The mean MI of cases in the age group 15–45 years is 58.973. Out of three cases that were under 15 years old, there was only one case with a midzone shift and two cases with the right zone shift. The mean MV of cases under 15 years is 68.667. Out of five cases in the above 45 years age group, there were only three cases of which two showed left zone shift. One case showed a mid-zone shift, and 1 case showed a right-zone shift. The mean MI of cases in age above 45 years is 32.6.
Correlation with manner of death
The different manners of death were divided into suicide, accident, natural, cannot be determined, and homicide. Out of a total of 64 cases studied, 48 cases (75%) were suicidal, and 16 (25%) were non-suicidal in manner. Non-suicidal cases comprised ten accidentals, three natural, and one homicidal manner of death, and in two cases, the manner of death could not be determined. Twenty-six cases out of 48 suicidal showed a mid-zone shift of MI, which means that intermediate cells were predominant, indicating a late luteal phase; 17 suicide cases showed a right zone shift and five suicidal cases showed a left zone shift of MI. The mean MV of the 48 suicidal cases is 60.615. The remaining 16 cases were non-suicidal, of which six cases show mid-zone shift and the remaining ten cases show right and left zone shift of MI. Suicide was the most common manner of death seen.
A one-way analysis of variance test was used to compare the means of MV in different manners of deaths, and the P-value was found to be 0.045, which is significant [Table 2]. When the same analysis was done for a different age group, the P-value was found to be 0.071, which is not significant [Table 3].
| Manner of death | n | Mean MV | Std. Deviation of MV | Std. Error | 95% Confidence interval for mean | P-value | |
|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||||
| Suicide | 48 | 60.615 | 22.7262 | 3.2802 | 54.016 | 67.214 | 0.045 |
| Accident | 10 | 40.1 | 25.9763 | 8.2144 | 21.518 | 58.682 | |
| Natural | 3 | 40 | 50.4306 | 29.1161 | −85.277 | 165.277 | |
| Cannot determine | 2 | 71 | 36.7696 | 26 | −259.361 | 401.361 | |
| Homicide | 1 | 99 | . | . | . | . | |
| Total | 64 | 57.36 | 26.2115 | 3.2764 | 50.82 | 63.915 | |
MV: Maturation value
| Age | n | Mean MV | Std. Deviation of MV | Std. Error | 95% Confidence interval for mean | P-value | |
|---|---|---|---|---|---|---|---|
| Lower Bound | Upper bound | ||||||
| <15 years | 3 | 68.667 | 9.8277 | 5.674 | 44.253 | 93.08 | 0.071 |
| 15–45 years | 56 | 58.973 | 24.9327 | 3.3318 | 52.296 | 65.65 | |
| Above 45 years | 5 | 32.6 | 36.4201 | 16.2876 | −12.622 | 77.822 | |
| Total | 64 | 57.367 | 26.2115 | 3.2764 | 50.82 | 63.915 | |
MV: Maturation value
DISCUSSION
Vaginal cytology is a quick, affordable, simple, and effective tool for determining the hormonal state and related disorders. To evaluate the ovarian function of adolescence, the reproductive age group, menopause, and old age, vaginal cytology is helpful and helpful in monitoring outcomes of hormonal therapy. However, cytomorphological study is rarely done to understand the hormonal profile in female autopsies. A postmortem study of vaginal cytology is done to identify evidence indicative of sexual assault and plays an important role in the investigation of suspicious female deaths. The importance should not only be limited to sexual assault investigation but also help in understanding the hormonal profile of women, which can help us to understand the possible psychological status and its relationship with the manner of death.
In our study, the samples were collected during the medicolegal autopsy with a postmortem interval of 6–48 h for the initial purpose of ruling out the presence of spermatozoa, and these slides were included in this study. Although many researchers have advocated immediate wet fixation of vaginal smears before staining;[12,13] in our study, we used the air-dried smears before fixation and got good results.
Mono-hormonal effect of estrogen includes proliferation, differentiation, and maturation of stratified squamous epithelium. Estrogen causes the maturation of cells.[13-18] Progesterone causes regression of pyknotic superficial cells to intermediate cells. Heavy exfoliation of intermediate cells is seen in the progesterone-predominant phase of the menstrual cycle.[1] Even though vaginal cytologic traits have been researched all over the world, this topic has gotten little attention. In our study, 56 cases were in the reproductive age group, with 31 cases showing a mid-zone shift of MI. The average MV of total cases was 57.36. About 75% (48 out of 64) cases were suicidal, with an average MV of 60.61, and 54.2% of suicidal cases show a mid-zone shift, indicating predominantly intermediate cells, which are possible in the late luteal phase of the menstrual cycle. According to a study done by Devi et al.,[17] vaginal swabs taken during the follicular phase were predominantly of superficial and intermediate cells. The luteal phase was predominantly intermediate cells with folding and crowding. The karyopyknotic index was highest during the follicular phase when the estrogen level was high. There are studies demonstrating the association of suicidal behavior in women with PMDD [8-11], and it occurs in the luteal phase of the menstrual cycle. Mousavi et al.[19] studied the estrogen and progesterone serum levels of the 111 females, which were measured 24 h after a suicidal attempt. The mean blood concentrations of progesterone and estrogen were 2.99 ng/mL and 76.8 pg/mL, respectively. They reported that of them, 62.2% were in the luteal phase, and 37.8% were in the follicular phase. The patients with more than two prior attempts at suicide had considerably greater serum progesterone concentrations than the other individuals. This can be correlated with the present study, which has maximum suicidal cases with mid-zone shifts showing predominantly intermediate cells indicative of the luteal phase.
PMDD is characterized by severe physical and psychological changes that occur during the luteal menstrual phase.[20-23] Some women experience changes in suicidal behavior and risk factors that are related to the menstrual cycle, indicating that hormone fluctuations during the menstrual cycle may be one predictable time-varying trigger for sudden increases in suicide risk.[9] In a study done by histopathological examination of uterine tissue, deaths were more common during the secretory phase (56.9%) in the suicidal group, while in the non-suicidal group, death occurred more commonly in the proliferative phase (66.3%).[18] The cytological profile of the present study reflects the luteal phase, which corresponds to the secretory phase of the uterine cycle.
Limitations
Menstrual history, history of pregnancy/childbirth, medication, and symptoms were not taken. Body mass index was also not considered.
CONCLUSIONS
In this study, most of the cases were in the reproductive age group. There was a maximum midzone shift among suicides. The cellular picture reflects more suicidal cases in the luteal phase of the menstrual cycle. This corroborates with the findings of several studies where suicidal risk behavior was found to be associated with PMDD, which happens in the luteal phase of the menstrual cycle. However, it is best to correlate the cytological profile with the menstrual and pregnancy history. The study of vaginal cytology is not limited to the identification of evidence in sexual offense cases. Vaginal cytology is a reliable study to identify the possible hormonal status and may help to correlate the suicidal risk behaviors in such cases, especially where the manner of death is unknown or suspicious.
Ethical approval
The research/study was approved by the Institutional Review Board at All India Institute of Medical Sciences Bhopal, number IL-095, dated April 13, 2023.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Cyto-hormonal profile of a female from birth to postmenopausal phase In: A review (1st ed). India: CBS Publishers and Distributors Pvt Ltd; 2017.
- [Google Scholar]
- The fate of ovaries preserved at the time of hysterectomy. Am J Obstet Gynecol. 1966;96:1088-97.
- [CrossRef] [PubMed] [Google Scholar]
- Exfoliative cytology in gynaecological practice. Postgrad Med J. 1966;42:283-4.
- [CrossRef] [Google Scholar]
- Post-menopausal smear patterns--a review of vaginal smears in 480 women. Med J Malaysia. 1992;47:38-43.
- [Google Scholar]
- Further menstrual characteristics of suicide attempters. J Psychosom Res. 1975;19:81-5.
- [CrossRef] [PubMed] [Google Scholar]
- Premenstrual syndrome: A mini review. Maturitas. 2015;82:436-40.
- [CrossRef] [PubMed] [Google Scholar]
- Suicidal risk in women with premenstrual syndrome and premenstrual dysphoric disorder: A systematic review and meta-analysis. J Womens Health (Larchmt). 2021;30:1693-707.
- [CrossRef] [PubMed] [Google Scholar]
- Suicidality in women with premenstrual dysphoric disorder: A systematic literature review. Arch Womens Ment Health. 2021;24:173-84.
- [CrossRef] [PubMed] [Google Scholar]
- Understanding menstrual cycle effects on suicide will require prospective studies of suicidal thoughts and behaviors in premenstrual disorders. BMC Med. 2021;19:135.
- [CrossRef] [PubMed] [Google Scholar]
- Association between suicidal behavior and clinical features of premenstrual syndrome and menstrual history: A cross sectional study. J Clin Med Res. 2018;10:830-7.
- [CrossRef] [PubMed] [Google Scholar]
- Suicidal behaviour and the menstrual cycle. Psychol Med. 2006;36:901-12.
- [CrossRef] [PubMed] [Google Scholar]
- Pap smear collection and preparation: Key points. Cytojournal. 2022;19:24.
- [CrossRef] [PubMed] [Google Scholar]
- A simple technique for preparing vaginal smears. Am J Obstet Gynaecol. 1945;50:565-7.
- [CrossRef] [Google Scholar]
- The role of vaginal maturation value assessment in prediction of vaginal pH, serum FSH and E2 levels. Marmara Med J. 2006;19:52-7.
- [Google Scholar]
- Prevalence and correlates of vaginal estrogenization in postmenopausal women in the United States. Menopause. 2017;24:536-45.
- [CrossRef] [PubMed] [Google Scholar]
- Deception and vaginal slides: Do we need to preserve a control sample of victims in every case of rape? J For Med Leg Aff. 2015;1:103.
- [CrossRef] [Google Scholar]
- A 3 years study of vaginal hormonal cytology at tertiary hospital. IAIM. 2017;4:20-32.
- [Google Scholar]
- Cytohormonal and morphological alterations in cervicovaginal smears of postmenopausal women on hormone replacement therapy. Diagn Cytopathol. 2006;34:676-81.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrent suicide attempt and female hormones. Adv Biomed Res. 2014;3:201.
- [CrossRef] [PubMed] [Google Scholar]
- Association of menstruation cycle with completed suicide: A hospital-based case-control study. Arch Womens Ment Health. 2019;22:771-7.
- [CrossRef] [PubMed] [Google Scholar]
- Suicidal ideas during premenstrual phase. J Affect Disord. 1995;34:193-9.
- [CrossRef] [PubMed] [Google Scholar]
- Personality traits of suicidality are associated with premenstrual syndrome and premenstrual dysphoric disorder in a suicidal women sample. PLoS One. 2016;11:e0148653.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence, correlates, comorbidities, and suicidal tendencies of premenstrual dysphoric disorder in a nationwide sample of Korean women. Soc Psychiatry Psychiatr Epidemiol. 2012;47:1937-45.
- [CrossRef] [PubMed] [Google Scholar]






