Translate this page into:
Rapid Identification and Drug Susceptibility Testing of Mycobacterium tuberculosis: Standard Operating Procedure for Non-Commercial Assays: Part 3: Colorimetric Redox Indicator Assay v1.3.12
Address for correspondence: Prof. Sarman Singh, E-mail: sarman_singh@yahoo.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The previous two standard operating procedures (SOPs) related to the culture and drug susceptibility testing (DST) of Mycobacterium tuberculosis with the microscopic observation drug susceptibility assay (Part 1) and nitrate reductase assay (Part 2). The present SOP is devoted to a third non-commercial culture and DST method known as colorimetric redox indicator assay (CRI). As its name indicates, the CRI detects the ability of the M. tuberculosis to reduce the colored oxidation-reduction indicator when added to a liquid culture of M. tuberculosis, after exposing the growth to different anti-mycobacterial drugs. The change in the color of the indicator denotes the proportionate number of viable Mycobacteria in the medium. The identification and DST results can be obtained in 7-8 days. This SOP document has been developed through the culture and DST subgroup of the STOP tuberculosis (TB) Partnership New Diagnostic Working Group. It is intended for laboratories that would want to use or already use this rapid non-commercial method for culture identification and DST of M. tuberculosis, notably in resource-constraint settings in Asia and Africa.
Keywords
Mycobacterium
tuberculosis
drugs
susceptibility
testing
training
INTRODUCTION
Scope
This standard operating procedure (SOP) document has been specially compiled for the implementation of non-commercial culture and drug susceptibility testing (DST) methods endorsed by New Diagnostic Working Group-STOP tuberculosis (TB) Partnership (WHO) for laboratory network performing the rapid non-commercial rapid culture identification and DST testing for Mycobacterium tuberculosis and intended for the use of TB diagnostic laboratories, located in various Asian countries. It is also intended as a companion to the TB laboratory training manuals.
This SOP describes the procedure for the colorimetric redox indicator (CRI) assay methods for M. tuberculosis isolates grown from conventional culture. This indirect method is based on the reduction of a colored indicator added to liquid culture medium on a microtiter plate after in vitro exposure ofM. tuberculosis isolates to anti-TB drugs. The time for diagnosis of multidrug resistant ( MDR) is faster than other conventional phenotypic DST methods in liquid culture. The rapid detection of M. tuberculosis and information about DST allows proper management of TB.
As suspensions with viable, infectious bacteria are handled, strict compliance with safety and protection measures is mandatory. The procedure must be carried out in a laboratory, meeting WHO standards for biosafety level two with access restricted to authorized personnel only.
Purpose
The purpose of this SOP is to rapidly provide clinicians with information about the patients suspected to have MDR M. tuberculosis for proper clinical management.
Personnel qualifications
The test performer should be having a diploma in laboratory technologies and preferably university graduate in biological sciences with sufficient experience.
Medical fitness
In accordance with national laws and practices, arrangements should be made for an appropriate health surveillance of TB laboratory workers:
-
Before enrolment in the TB laboratory
-
At regular intervals thereafter, annually or bi-annually
-
After any biohazard incident
-
In case of onset of TB symptoms
All cases of disease or death identified in accordance with national laws and/or practice as resulting from occupational exposure to biological agents shall be notified to the competent authority.
Education and training
Personnel are required to be knowledgeable about the procedures in this SOP. Documentation of training and familiarization with this SOP can be found in the training file for each employee.
The laboratory staff shall confirm (i.e., documentation in the training file of familiarization with the SOP) that they can properly perform the procedure before commencing work. Education and training must be given on the following topics:
-
Potential risks to health (symptoms of TB disease and transmission)
-
Precautions to be taken to minimize aerosol formation and prevent exposure
-
Hygiene requirements
-
Wearing and use of protective equipment and clothing
-
Handling of potentially infectious materials
-
Laboratory design, including airflow conditions
-
Use of biological safety cabinets (BSCs) (operation identification of malfunctions, maintenance)
-
Use of autoclaves, incubators (operation, identification of malfunctions, maintenance)
-
Prevention of incidents and steps to be taken by workers in case of incidents (biohazard incidents, chemical, electrical and fire hazards)
-
Good laboratory practice and good microbiological techniques
-
Organization of workflow and procedures
-
Waste management
-
Importance of laboratory results for patient management
-
Importance of laboratory results for the national TB program
-
Training shall be given before a staff member takes up his or her post
-
Repeat training periodically, preferably every year
Bio-safety precautions in tuberculosis laboratory
TB laboratory should have all the major requirements and facility for handling M. tuberculosis safely, and involves minimum risk to the laboratory personnel, if they take proper precautions, and employ proper techniques described in these SOPs. Laboratory safety involves all the procedures and methods one needs to follow to minimize the risks of laboratory acquired infections. Use of laboratory is limited to trained TB lab personnel.
Biological safety cabinets
-
Switch ON the safety cabinets for at least 30 min before use. Note that the reading on the mini gauge pressure is satisfactory
-
Wear double pair of gloves, every time you work inside the cabinet
-
Biosafety cabinets need to be cleaned with 5% phenolic or 1% hypochlorite solution before work
-
Keep disposal bin/vessel with 5% phenolic or 5% hypochlorite disinfects inside the cabinet at the right side corner
-
Wipe of gauge-cloth soaked in 5% phenolic or 5% hypochlorite, should be readily available inside the cabinet
-
Arrange all un-infected material required towards the left side
-
All the processed samples need to be arranged right side
-
Do not process more than six specimens at a time, inside the cabinet.
-
After completion of work, wipe off the surface with 5% phenolic solution, and discard all wipes in biohazard bags, or in disposal container meant for infectious materials
-
Discard off the outer glove, too, inside the bio-safety cabinet
-
Wipe off the inner glove with disinfectant before touching anything else in the lab
Waste disposal and handling
All infectious waste should be discarded in the bio-safety disposal bin. All infectious solid waste-wipes, swabs, plastic, paper towels, gauze pads, gloves, etc., should be placed inside the double autoclave bags, sealed with autoclave tape and sterilized at 121°C for 30 min in the autoclave.
Liquid waste, in the steel discarding bins, should be disinfected in 5% phenol for at least 1 h, before sealing the caps and autoclaved at 121°C for 30 min.
Accidents and spillages
-
Spills inside BSC
All workers using the BSC should keep absorbent materials (gauge cloth/adsorbent sheet) and 5% phenol within the cabinet.
-
Alert all people in the adjacent laboratories in the event of spill
-
Spread 5% phenol soaked wipe immediately, while the BSC continues to operate. Wait for 15-20 min.
-
Use paper towels to wipe up the spill, working from the edges into the center
-
Decontaminate equipment: Items that are not readily or easily surface decontaminated should be carefully placed into autoclave bags and removed for further treatment (e.g., decontamination by autoclaving)
-
Contaminated gloves and clothes (sleeves are most likely to be contaminated); remove and decontaminate the lab coat by autoclaving or soaking in decontaminant.
-
-
Spills outside containment room in the BSC
Spills on equipment (such as vortex, centrifuge, incubator, refrigerator etc.), laboratory benches, walls or floors:
-
Immediately indicate to all personnel working in the lab, and evacuate for 1 h to allow dissipation of aerosols created by the spill (negative air pressure system would clear the aerosols)
-
Leave the BSC operating and cultures inside cabinet
-
Leave the containment facility following exit procedures
-
Close laboratory doors and post warning signs to prevent others from entering the laboratory
-
Thoroughly wash hands and other apparently contaminated areas with soap and water. Put on clean disposable gloves
-
If personal clothing is contaminated, remove all outer clothing and place it in the autoclave or container for autoclaving. Put on clean garments
-
Upon returning to the laboratory wear the N95 mask, fresh lab coat and double pair gloves to start decontamination, cover the spill area with paper towels soaked in 5% phenol solution or 1:10 dilution of 20% bleach (freshly prepared), or 70% ethanol solution (Do not pour decontamination solution directly onto the spill in order to avoid additional release of aerosols)
-
Let it stand for 20 min then wipe up with paper towels
-
Wipe up the spill with the soaked paper towels and place the used towels in an autoclave bag and autoclave
-
Place gloves and paper towels in autoclave bag and autoclave
-
Spill inside the centrifuge bucket/tube: Always use the aerosol containment cups for centrifuging. Always open the centrifuge buckets inside the bio-safety cabinet. Autoclave the buckets
-
Wash hands and other apparently contaminated areas again with soap and water
-
-
Dont's
-
Eating, drinking, smoking, applying cosmetics, use of mobile phones, or applying contact lenses in the TB laboratory
-
Don't allow unauthorized personnel to enter the TB laboratory
-
Mouth pipetting
-
Crowding of lab with material that is not required inside
-
PROCEDURE
Principle
Colorimetric methods for detecting drug resistance in M. tuberculosis are based on the reduction of an oxidation-reduction indicator added to a liquid culture medium after M. tuberculosis has been exposed in vitro to different antibiotics. Resistance is detected by a change in color of the oxidation-reduction indicator, which is directly proportional to the number of viable mycobacteria in the medium.
Samples
Pure cultures of acid - fast bacilli grown on solid media and liquid medium are used for indirect microscopic observation drug susceptibility assay.
Confirm the growth as M. tuberculosis using mycobacterial antigen 64(MPT64), p-nitro benzoic acid (PNB) or any other standard methods being practiced in the local laboratory.
Equipment and materials
-
7H9 broth: Middlebrook 7H9 (Becton Dickinson ref. 271310)
-
Oleic acid dextrose catalase (OADC) (10 × 20 ml Becton Dickinson ref. 211886)
-
Glycerol (Merck ref. 356350)
-
Casitone (pancreatic digest casein Bacto Casitone 225930 BD)
-
96-well plate with lid: (box of 50 plate-Becton Dickinson ref. 353072)
-
Filter 0.2 μm: Acrodisc Syringe filter (50 filters-Pall Life Sciences ref. 4652)
-
Syringes for filter: 2 or 5 ml
-
Distilled water (DW)
-
37°C incubator
-
Micropipettes: 1000 μl/200 μl
-
Tips + tips with filter (1000 μl/200 μl)
-
Multichannel pipette
-
Resazurin dye: Resazurin sodium salt (Sigma ®, USA, ref. R 7017)
-
Analytic balance
-
Incubator 37°C
-
Autoclave
-
Drugs for MDR screening: Rifampicin (RIF) (Sigma, ref. R7382), i soniazid (INH) (Sigma, ref. I3377)
Reagents and solutions preparation
-
7H9 medium with supplement (7H9-S) = 7H9 broth +10% OADC +0.5% glycerol +0.1% casitone
-
For 200 ml of 7H9-S medium:
-
Weigh 0.94 g of 7H9 powder and dissolve in 180 ml of DW; mix until complete solubilization
-
Weigh 0.2 g of casitone and add to the previous solution until complete solubilization. Warm the solution if necessary
-
Autoclave the broth in a 250 ml flask
-
After autoclaving and cooling, add 20 ml of OADC enrichment and 1 ml of sterile glycerol. Mix well
-
Check in the incubator for sterility (leave one night in the incubator and check the day after if there is no turbidity and if the media is still transparent)
-
Store the medium protected from direct light at 4°C
-
Drug stock solutions
-
INH: Weigh 2 mg of INH powder and dissolve in 2.0 ml of DW, making a stock solution of 1 mg/ml. Sterilize by filtration with a 0.22 μm syringe filter; aliquot (30 μl) and store frozen (−20°C) until use.
-
RIF: Weigh 20 mg of RIF powder, dissolve in 2.0 ml of absolute methanol, making a stock solution of 10 mg/ml. Sterilize by filtration with a 0.22 μm syringe filter; aliquot and store frozen (−20°C) until use (300 μl) [Table 1].

Drug working solutions
For the preparation working solutions of drugs, take the amount of drug stock solution and dilute into 7H9-S medium as per the amount given in Table 1.
Drugs storage
Drug solutions may be frozen in aliquots at −20°C and stored for up to 3 months. Once thawed, discard the leftover and do not store or refreeze. To avoid wastage of drugs during the freeze/thaw processes make the drugs aliquot as per the amount required at a time.
Preparation of inoculum
Inoculum from growth on solid medium
It is very important to have fresh growth on a solid medium. Older cultures may result in unreliable susceptibility test results.
-
Take a loop full of bacterial growth with a sterile loop and put it in a sterile vial with glass beads just covering the bottom of the vial with 2.5 ml of 7H9-S broth (try not to take any medium when removing growth)
-
Vortex the vial for at least 1 min to break the clumps until a fairly turbid suspension is obtained
-
Allow it to stand for 5 min
-
Transfer the supernatant to a new sterile vial and leave it to sediment for 15 min
-
Transfer the supernatant to a new sterile vial
-
Compare the turbidity of the suspension using the scale McFarland 1.0 standard
-
Set the turbidity of inoculum as per McFarland 1.0 standard using 7H9-S
-
Make a 1:20 dilution of inoculum with 7H9-S broth
Inoculum from a liquid medium
-
Transfer 2.5 ml of the pellet of culture to a sterile vial containing glass beads
-
Vortex for at least 1 min until a fairly turbid suspension is obtained
-
Allow it to stand for 5 min
-
Transfer the supernatant to a new sterile vial and leave it to sediment for 15 min
-
Transfer the supernatant to a new sterile vial
-
Compare the turbidity of the suspension using the scale McFarland 1.0 standard
-
Set the turbidity of inoculum as per McFarland 1.0 standard using 7H9-S
-
Make a 1:20 dilution of inoculum with 7H9-S broth
Colorimetric redox indicator assay plate setting up for the multidrug resistant screening
Refer to the diagram of the 96 well microtiter plate [Figure 1].
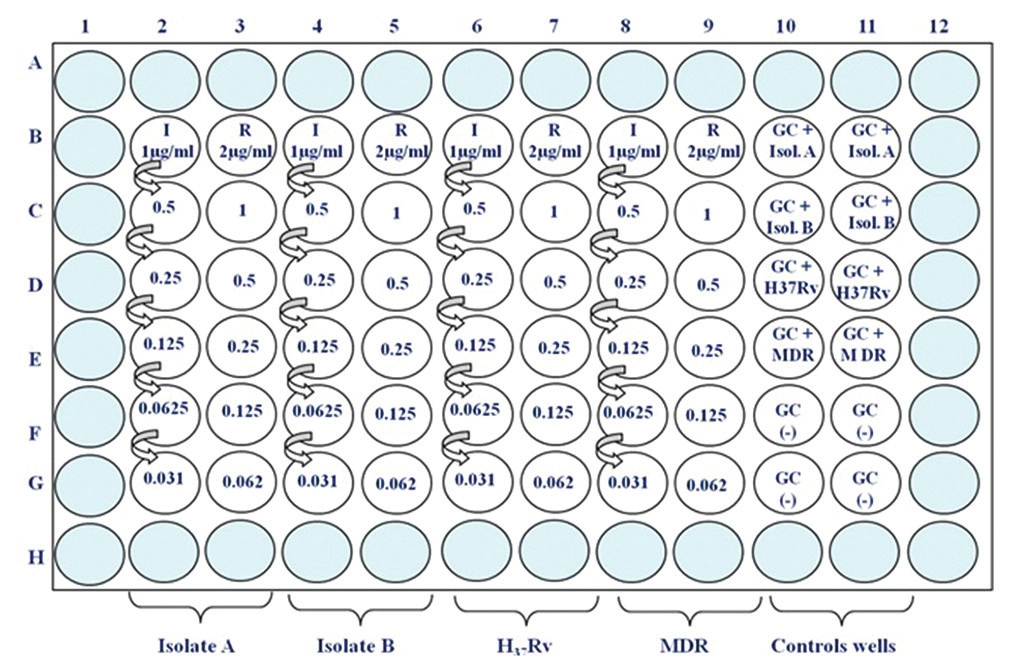
- Colorimetric redox indicator assay plate setting up for first line drugs (I: Isoniazid and R: Rifampicin) GC positive control can be done in duplicate for each isolate (Isol B10 and B11: +Isol A; C10 and C11: GC+Isol. B; D10 and D11 G+H37 Rv; E10 and E11 GC+ Multi drug resistant, F10-F11 will be the GC (-)
In a 96-well flat-bottom microtiter plate (with cover, BD) there is enough space to test four isolates against the two drugs- INH and RIF, in six 2-fold dilutions of each drug.
-
Add 100 μl of 7H9-S broth to all wells of the columns 2-11 from rows B to G
-
Add 100 μl of the working solution of INH to wells B2, B4, B6, and B8
-
Add 100 μl of the working solution of RIF to wells B3, B5, B7, and B9
-
With a multi-channel pipette make two fold serial dilutions (transfer 100 μl) from rows B to G (columns 2-9) discarding the last 100 μl after mixing in row G
-
Add 100 μl of 7H9-S broth to wells B11-E11; these will represent the negative and sterility controls on
-
the test
-
Inoculate plate with 100 μl of final culture suspension in respective culture wells including its respective growth control (GC) (+)
-
Add 200 μl of sterile DW to all the outer wells left without broth; these will prevent evaporation during incubation of the plate.
-
Cover the plate with lid, seal properly with parafilm from all sides or in zip lock bags and incubate at 37°C for 7 days. (Note: For each batch 1 set of quality control (QC) (1 H37Rv and 1 MDR) is run; if QC is not being run on the same plate in that case replace H37Rv and MDR with isolate C and D, respectively)
Preparation of resazurin dye
Prepare a resazurin solution at 0.02% in sterile DW, sterilized by 0.2 μm syringe filter. The dye solution must be stored in dark at 4°C for 2 weeks.
Color development and interpretation of results
After 7 days incubation, the plates are taken from the incubator and 30 μl of the resazurin dye solution is added to all the wells and the plate again sealed and incubated overnight for color development.
A change in color from blue to pink means a growth of the isolates at that concentration of the drug. For better interpretation of the results, the color must be compared to the color present in the GC well.
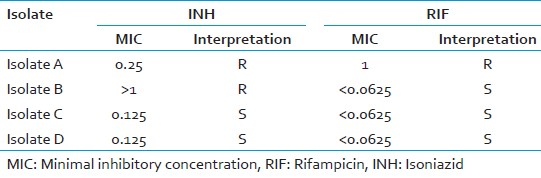
The minimal inhibitory concentration
Minimal inhibitory concentration (MIC) of each drug is interpreted as the lowest concentration of the antibiotic that prevents a change in color of the resazurin dye. MIC values are scored for each isolate for comparison with the results obtained with the proportion method.
Positive and negative controls
The positive control should show positive growth and the negative control (NC) should show no growth within the incubation protocol period. If NC shows a growth, investigate procedures, could be a cross-manipulation and check all reagents for possible source of contamination.
Breakpoint concentration
The isolate is considered as resistant isolate, which showed MIC values either equal to or higher than the given in Table 2 against the INH and RIF drugs.
Refer to the [Figure 2] and [Table 3] for the better interpretation of the results [Table 2].
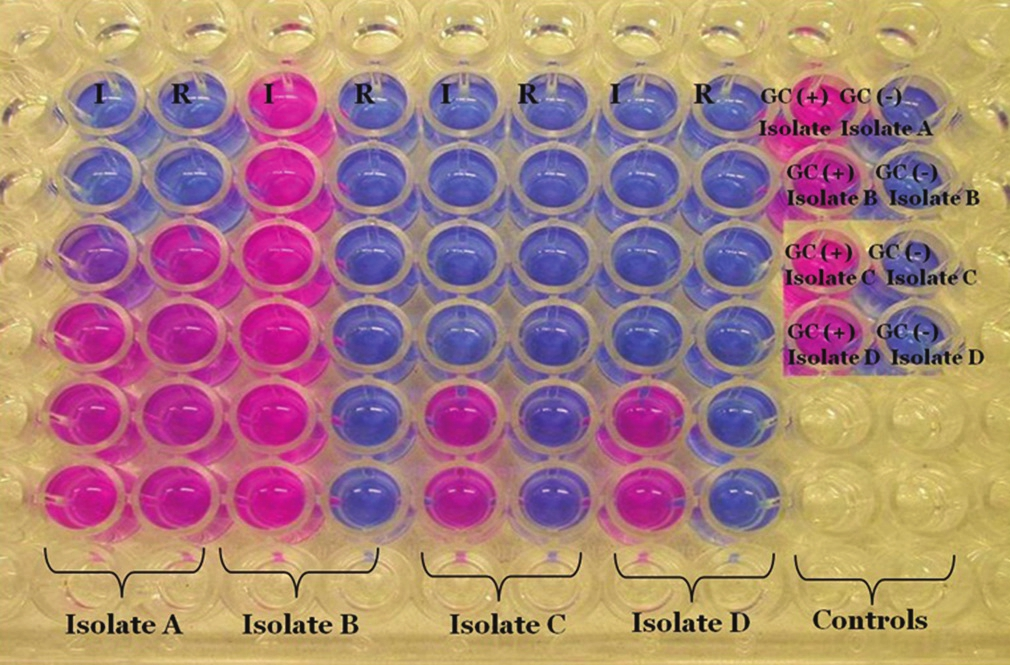
- Demonstration of colorimetric redox indicator assay plate, refer to Table 3 for result interpretation

Detection of contamination
The incidence of contamination varies from laboratory to laboratory depending on several factors. The recommendation is that up to 5% contamination rate is acceptable. Liquid media are more susceptible to contamination than solid media. It is extremely important to take care during the manipulation and to work with sterile material.
-
Any well with a turbid appearance is suspected of contamination and result of this well is not valid
-
At the moment that you add the resazurin dye if you observe directly a change of color the well could be contaminated and has to be discarded
Precautions
-
All work should be carried out in a proper BSC (class II)
-
All materials should be sterilized by autoclaving prior to disposal
-
Plate should be kept in the incubator in a box to avoid that someone moved the plate by accident
-
Plate should be closed on both sides by a small tape for security
Quality control
It is important to perform a quality control (QC) of DST for first-line drugs testing. Add the standard isolate of M. tuberculosis (H 37Rv). Preferably, a drug-resistant strain should also be included into the QC. If the susceptible H37Rv shows some resistance, then all the results obtained within during the experiment become invalid and the test should be repeated.
FURTHER READING
-
Martin A, Palomino JC. Procedure manual colorimetric redox indicator (CRI) for drug susceptibility testing forM. tuberculosis [Internet], version 06-2012. Available from: http://www.tbevidence.org. [Last accessed date on 4 Feb 2012]
-
Martin A, Morcillo N, Lemus D, Montoro E, Telles MA, Simboli N, et al. Multicenter study of MTT and resazurin assays for testing susceptibility to first-line anti-tuberculosis drugs. Int J Tuberc Lung Dis 2005;9:901-6.
-
Martin A, Portaels F, Palomino JC. Colorimetric redox-indicator methods for the rapid detection of multidrug resistance in Mycobacterium tuberculosis: A systematic review and meta-analysis. J Antimicrob Chemother 2007;59:175-83.
-
Sankar MM, Gopinath K, Singla R, Singh S. in-vitro antimycobacterial drug susceptibility testing of non-tubercular mycobacteria by tetrazolium microplate assay. Ann Clin Microbiol Antimicrob 2008;7:15.
-
World Health Organization. Non-commercial culture and drug-susceptibility testing methods for screening of patients at risk of multi-drug resistant tuberculosis: Policy statement, July, 2010. Available from: http://www.who.int/tb/dots/laboratory/whopolicy_noncommercialculture_and_dstmethods_july10.pdf. [Last accessed date on 11 Nov. 2012]
ACKNOWLEDGMENT
The authors would like to thank Dr. Alessandra Varga of FIND for her administrative support to this project, to Dr. David Moore for his technical inputs in the preparation and finalization of this SOP and all core members of the new diagnostic working group (NDWG) for approving this project.
Source of Support: Culture and drug susceptibility testing subgroup, Stop-TB Partnership, WHO & FIND, Geneva, Switzerland
Conflict of Interest: None declared.





