Translate this page into:
Platelet volume indices as predictive biomarkers for diabetic complications in Type 2 diabetic patients
Address for correspondence: Dr. Archana Buch, B 603, Gold Coast, Ivory Estates, Someshwarwadi, Pune - 411 008, Maharashtra, India. E-mail: drarchanabuch@yahoo.co.in
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
BACKGROUND:
Platelet volume indices (PVI) such as mean platelet volume (MPV), platelet distribution width (PDW), and platelet-large cell ratio (P-LCR) are the indicators of increased platelet activity and can be considered as potential biomarkers for diabetic complications.
PURPOSE:
To study PVI in Type 2 diabetics with and without complications in comparison to nondiabetic patients.
MATERIALS AND METHODS:
A case–control study was conducted on 300 Type 2 diabetics and 200 nondiabetics. Detailed clinical history regarding duration, hypertension, and complications was taken. PVI was obtained using automated cell counter. Fasting blood glucose, hemoglobin A1c, lipid profile, creatinine were also obtained. Diabetics were further categorized into patients with complications and without complications. Statistical analysis was performed by Statistical Package for the Social Sciences Version 17 (Chicago, IL) Student's t-test and ANOVA test.
RESULTS:
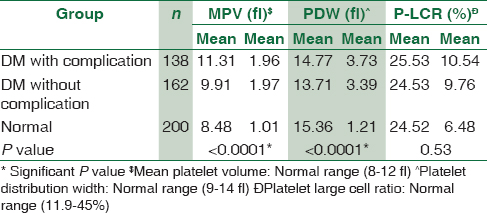
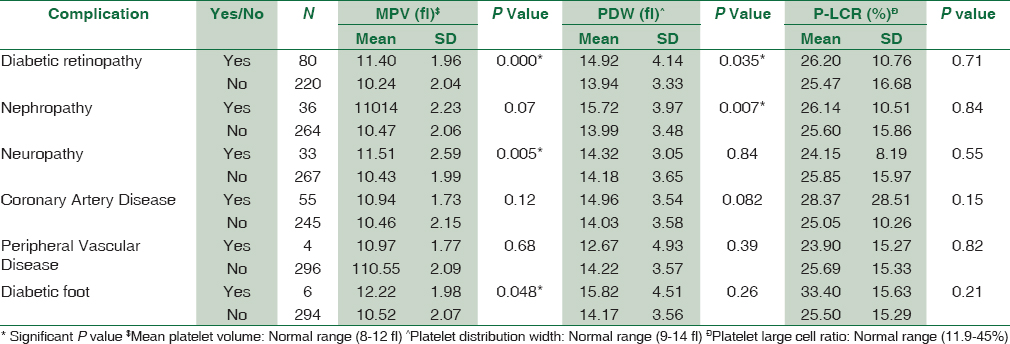
Platelet count was significantly decreased in diabetics (P = 0.005). MPV was significantly increased in diabetic patients with complications as compared to diabetics without complications and nondiabetic group (P < 0.0001). PDW showed statistically significant difference between diabetics with and without complications and nondiabetics (P < 0.0001). However, no statistically significant difference was observed in platelet-large cell ratio (P-LCR) among all the three study groups. We found statistically significant correlation of MPV with diabetic retinopathy (P = 0.000), nephropathy (P = 0.005), and diabetic foot (P = 0.048). PDW was significantly increased in diabetic retinopathy (P = 0.035) and nephropathy (P = 0.007). P-LCR had no statistically significant correlation with diabetic complications.
CONCLUSION:
MPV and PDW are predictive biomarkers of diabetic vascular complications. They are more significant in microvascular complications than macrovascular complications.
Keywords
Diabetes
mean platelet volume
platelet distribution width and platelet large cell ratio
platelet volume indices
Introduction
Diabetes mellitus (DM) is the most challenging problem in today's world.[1] It is a complex disease characterized by chronic hyperglycemia, metabolic abnormalities, and long-term macro- and micro-vascular complications involving the blood vessels, eyes, kidneys, and nerves.[2] Type 2 diabetes accounts for over 80% of cases of DM and is a slow onset, heterogeneous disorder resulting from interactions between environmental factors and polygenetic inheritance.[3]
Diabetic complications are mainly due to hyperglycemia and are responsible for the majority of morbidity and mortality associated with DM. Fasting blood glucose, postprandial blood glucose, and hemoglobin A1c (HbA1c) are widely used to monitor glycometabolic control in patients with DM. HbA1c is a more useful marker to determine mean blood glucose levels over a long time period.[4]
DM is considered as a “prothrombotic state” owing to sustained hyperglycemia, dyslipidemia, and insulin resistance causing endothelial and pericyte injury. Altered platelet morphology and function has been observed in diabetes in the form of enhanced platelet activity which may contribute to this “prothrombotic state”.[5] Larger platelets that contain denser granules are metabolically and enzymatically more active than smaller ones and have higher thrombotic potential. Hence, increased mean platelet volume (MPV) and platelet distribution width (PDW) might be linked with increased thrombotic potential.[6]
The association of increased MPV, PDW, P-LCR, and platelet count with diseases related to endothelial dysfunction such as metabolic syndrome, diabetes, coronary artery disease (CAD), and malignancy has been shown in many studies.[7891011]
The newer hematological analyzers are giving variety of platelet parameters which helps in easy detection of change in platelet structure, which may help in early detection of prothrombotic state of the platelets. These can act as an alarm for diagnosing initiation/progression of diabetic complications. Hence, in view of this, we aim to study platelet parameters in type 2 diabetes and its predictive role in diabetic angiopathies.
Materials and Methods
The study was conducted in a tertiary care hospital and research center in Maharashtra. The study included 300 diabetic patients and 200 nondiabetics without CAD. All cases and controls were recruited from the outpatient department of medicine over a period of 1 year. Institutional Ethical Committee clearance was obtained before the start of study. Informed and written consent of the patients was taken.
We included diabetic patients diagnosed in accordance with the American Diabetes Association.[12] The control group was obtained from individuals without DM, as obtained from their medical records. Females with Hb <10 g% and males Hb <12 g%, nondiabetic subjects with CAD, pregnant women, patients on antiplatelet drugs such as aspirin and clopidogrel and subjects with any diagnosed malignancy were excluded from the study.
All the diabetic and nondiabetic subjects underwent complete clinical evaluation with specific reference to any micro- and macro-vascular complications.
Blood sample was taken under all aseptic precautions from the antecubital vein by a clean puncture avoiding bubbles and froth. About 2 ml of blood sample was collected in EDTA, fluoride bulb, and plain bulb each. Complete hemogram was performed by using automatic blood counter Sysmex KX-21 and H51 Benesphera coulter from EDTA bulb. Hb, platelet count, MPV, PDW, and P-LCR were also recorded. Plasma glucose levels were measured by the glucose oxidase method. HbA1c level was analyzed by immunoturbidometric inhibition method. Total cholesterol, triglyceride (TG), and high density lipoprotein (HDL) was measured by commercial enzymatic calorimetric method and low density lipoprotein (LDL) by direct enzymatic method.
In addition, diabetic patients were also evaluated regarding various microvascular complications such as diabetic retinopathy, neuropathy, and nephropathy, and macrovascular complications such as CAD, peripheral arterial disease (PAD), and diabetic foot. CAD findings were based on the clinical symptoms, 2D echocardiography (ECHO) observations of the patients. PAD had been diagnosed on the basis of clinical symptoms, ability to walk distances, and Doppler ECHO study of lower limbs. Diabetic foot was diagnosed based on five criteria of the National Diabetes Education Program: Lack of protective sensation (sensory neuropathy), absent pedal pulses, foot deformity, current or past foot ulcer, and history of foot amputation.[13]
Retinopathy diagnosis was based on the findings of at least two microaneurysms and/or retinal damage in the records. The quantitative urine albumin/creatinine ratio in the morning spot urine samples, increased BUN and creatinine was used for standard diagnosis of diabetic nephropathy. Diabetic neuropathy was based on the clinical records available.
Statistical analysis was performed by Statistical Package for the social sciences (SPSS) version 17 (Chicago, IL). Student's t-test was used to find the significant difference of FBS, HbA1c, MPV, PDW, P-LCR according to complications, and ANOVA test was used to find the significant difference of MPV, PDW, P-LCR between all three groups. Data were expressed as mean ± standard deviation. A P < 0.05 was considered statistically significant.
Results
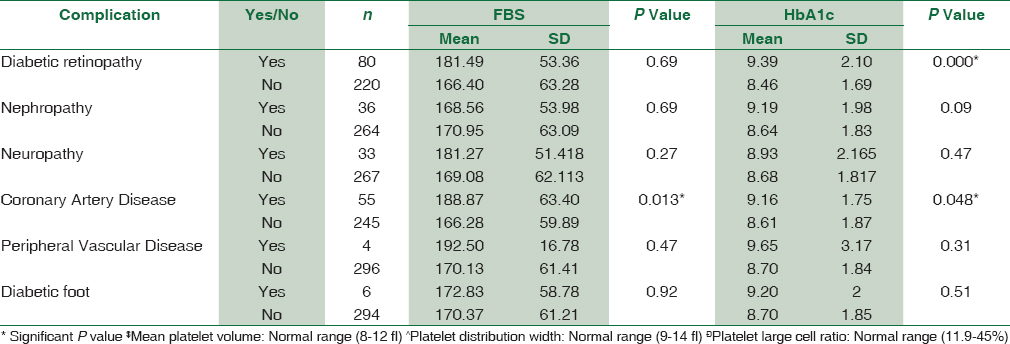
A total of 300 diabetic patients (198 males, 102 females) and 200 controls (120 males, 80 females) were selected for the study. Maximum number of diabetic males was in the age group of 51–60 years. Comparison of platelet count and platelet indices was made in cases and controls. The study suggested that MPV was significantly increased in diabetic patients with complications than diabetics without complications than nondiabetic group (P < 0.0001). There was a statistically significant difference of PDW between diabetics with complications, diabetics without complications and nondiabetic groups (P < 0.0001). However, no statistically significant difference was observed in P-LCR among all the three study groups [Table 1]. The study reinforced the fact that poor glycemic control and raised FBS causes increased the risk of diabetic complications [Table 2]. The association of platelet indices with various diabetic complications was shown in Table 3. We found statistically significant correlation between MPV and diabetic retinopathy (P = 0.000), nephropathy (P = 0.005), and diabetic foot (P = 0.048) and in rest of the complications, MPV is increased but not statistically significant. PDW was statistically significantly increased in diabetic retinopathy (P = 0.035) and nephropathy (P = 0.007) and it is statistically insignificant in rest of the complications. P-LCR had no statistically significant correlation with diabetic complications.
Platelet count was significantly decreased in diabetic cases (P = 0.005) Increased MPV was found to be statistically significant with duration of DM (P = 0.012), blood pressure (P = 0.007), and serum creatinine (P = 0.001). However, there was no statistically significant correlation of Platelet count, PDW, P-LCR with the duration of DM, blood pressure, and serum creatinine.
Platelet count had no statistically significant correlation with total cholesterol. Platelet indices increased with increased total cholesterol; however, it was statistically insignificant. TG showed statistically significant correlation with platelet count (P = 0.012), MPV (P = 0.000), PDW (P = 0.012). PDW was significantly increased with LDL levels (P = 0.007). However, platelet count, MPV, P-LCR had no statistically significant correlation with LDL and HDL levels.
Discussion
Diabetes is a growing health problem associated with increased risk of micro- and macro-vascular complications.[14] With the easy availability of various blood tests such as platelet volume indices (PVI), efforts are made to identify and prove their utility to act as biomarkers for early detection of diabetic complications.
We found that platelet count was decreased in diabetic patients as compared to control group. However, platelet count did not show any statistical significance on diabetic complications.[15] Insulin resistance and hyperglycemia are said to be the important factors causing increased platelet reactivity in diabetic patients. Platelet hyper reactivity is a well-known contributing factor to the prothrombotic state in diabetics, hence causing increased coagulation, impaired fibrinolysis, and endothelial dysfunction. These hyperactive platelets play a critical role in the pathophysiology of the thrombotic events leading to diabetic complications.[16]
MPV is a parameter used to assess platelet size, and it is a potential biomarker of platelet reactivity. It has been shown that larger platelets are more reactive than smaller ones.[17] PDW can directly measure the variability in platelet size, and its high values suggest increased production of larger reticulated platelets.[18]
Our study suggests that increased MPV is associated with poor glycometabolic control and it is also reflected in many complications such as retinopathy, nephropathy, CAD, and diabetic foot.
Several studies indicated positive correlation of FBS and HbA1c with platelet indices.[1920] However, some studies have not shown any relation of FBS and HbA1c with platelet indices.[2122] It has been proposed that increase in MPV could be because of raised blood sugar leading to osmotic swelling and shorter life span of platelets in diabetic patients. Alternatively, this may suggest that platelet activation is related to glycemic control.[23]
We found that duration of diabetes has a significant relation with increased MPV as agreed by others.[22] Discordant results were also found in other studies.[24] Platelet count, PDW, P-LCR had no statistically significant correlation with the duration of DM. Platelet number and reactivity along with the cardiovascular comorbidities such as hypertension, albuminuria, obesity, cigarette smoking, and dyslipidemia also contributes to the progression of diabetes and its effect on platelet indices. Thus, it shows that there are many other factors which may account for the thrombotic potential of diabetics with time.[25]
In our study, MPV showed statistically significant increase as the blood pressure increases which is consistent with previous study.[26] However, blood pressure had no statistically significant co-relation with platelet count, PDW, and P-LCR. MPV was not associated with change in systolic and diastolic BP in a study done by Dindar. Renin-angiotensin system blockade with medication and a well-controlled BP could be the reason given for this finding.[14]
In our study, we found positive correlation between PVI and cholesterol. MPV and PDW significantly increased with increase in TG and LDL as also reported by Ravindran and Krishnan.[27] Insulin resistance plays a pivotal role in the development of diabetic dyslipidemia by influencing several factors. In insulin resistance and Type 2 diabetes, increased efflux of free fatty acids from adipose tissue and impaired insulin mediated skeletal muscle uptake of free fatty acids increase fatty acid influx into the liver. A cluster of interrelated plasma lipid and lipoprotein abnormalities associated with alterations in very LDL metabolism contribute to the risk for atherosclerosis and CAD in the majority of patients with Type 2 diabetes. Insulin resistance plays a key role in the development of diabetic dyslipidemia.[28] Altered lipid metabolism plays a major pathophysiological role in diabetes, which can lead to the development of various diabetic complications.
We found statistically significant correlation of MPV with microvascular complications such as diabetic retinopathy and diabetic nephropathy;[2122] similarly higher values were also seen in the studies done by Dindar et al., Ates et al.[1429] MPV was also associated with retinal neovascularization of diabetic retinopathy. Platelets in diabetics are active and have increased aggregation because of dysregulated signaling pathway. This contributes to thrombus formation and microcapillary embolization. The release of constrictive, oxidative, and mitogenic substances such as platelet-derived growth factor and vascular endothelial growth factor accelerates the progression of local vascular lesions such as neovascularization of lens in diabetic retinopathy.
On the other hand, MPV was not significantly different in patients with these complications in studies done by Demirtunc et al.[19]
We also found that PDW was raised in diabetic retinopathy and nephropathy. It was also raised but not statistically significant in complications such as CAD and diabetic foot. P-LCR did not show any change in diabetic complications.
There were not much data on these newer biomarkers in the literature search. However, further prospective studies with larger sample size are required for identifying the utility of these markers to predict the diabetic disease burden, keeping all the compounding risk factors in mind, especially to predict the impact of PVI on diabetic complications.
Conclusion
We all are aware of the risk factors for diabetic complications such as duration, glycemic control, blood pressure, and dyslipidemia. We found increase in MPV and PDW in all these high risk groups. This implies that raised MPV and PDW can be considered as biomarkers for early detection of impending complication. We found that these platelet indices were more statistically significant in microvascular complications as compared to macrovascular complications. P-LCR did not have much statistical significance in predicting diabetic complications.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- An update on the etiology and epidemiology of diabetes mellitus. Ann N Y Acad Sci. 2006;1084:1-29.
- [Google Scholar]
- Diabetes mellitus. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, eds. Harrison's Principles of Internal Medicine (18th ed). New York: McGraw-Hill; 2012. p. :2968-3002.
- [Google Scholar]
- The pathophysiology of type 2 diabetes mellitus: An overview. Acta Physiol Scand. 2001;171:241-7.
- [Google Scholar]
- Standardization of HbA1c measurements – A consensus statement. Diabet Med. 2000;17:5-6.
- [Google Scholar]
- Mean platelet volume is an independent risk factor for myocardial infarction but not for coronary artery disease. Br J Haematol. 2002;117:399-404.
- [Google Scholar]
- Could mean platelet volume among complete blood count parameters be a surrogate marker of metabolic syndrome in pre-pubertal children? Platelets. 2014;25:393-8.
- [Google Scholar]
- Elevated mean platelet volume is associated with presence of colon cancer. Asian Pac J Cancer Prev. 2014;15:10501-4.
- [Google Scholar]
- The relationship between the plasma PCSK9 levels and platelet indices in patients with stable coronary artery disease. J Atheroscler Thromb. 2015;22:76-84.
- [Google Scholar]
- Platelet mean volume, distribution width, and count in type 2 diabetes, impaired fasting glucose, and metabolic syndrome: A meta-analysis. Diabetes Metab Res Rev. 2015;31:402-10.
- [Google Scholar]
- Plateletcrit: A novel prognostic marker for acute coronary syndrome. Int J Cardiol. 2014;177:161.
- [Google Scholar]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27:5-10.
- [Google Scholar]
- Effectiveness of foot care education among people with type 2 diabetes in rural Puducherry, India. Indian J Endocrinol Metab. 2014;18:106-10.
- [Google Scholar]
- Mean platelet volume is associated with glycaemic control and retinopathy in patients with type 2 diabetes mellitus. West Indian Med J. 2013;62:519-23.
- [Google Scholar]
- Hematological parameters and prediabetes and diabetes in adults from the general population. J Diabetes Metab. 2014;5:335.
- [Google Scholar]
- Mean platelet volume as a predictor of cardiovascular risk: A systematic review and meta-analysis. J Thromb Haemost. 2010;8:148-56.
- [Google Scholar]
- Effects of sorbinil treatment on erythrocytes and platelets of persons with diabetes. Diabetes Care. 1986;9:36-9.
- [Google Scholar]
- The relationship between glycemic control and platelet activity in type 2 diabetes mellitus. J Diabetes Complications. 2009;23:89-94.
- [Google Scholar]
- The relationship between diabetes, metabolic syndrome, and platelet activity as measured by mean platelet volume: The National Health and Nutrition Examination Survey, 1999-2004. Diabetes Care. 2012;35:1074-8.
- [Google Scholar]
- The relationship between mean platelet volume with microalbuminuria and glycemic control in patients with type II diabetes mellitus. Platelets. 2012;23:475-80.
- [Google Scholar]
- Elevated concentrations of soluble adhesion molecules and large platelets in diabetic patients: Are they markers of vascular disease and diabetic nephropathy? Clin Appl Thromb Hemost. 2007;13:391-7.
- [Google Scholar]
- The mean platelet volume in subjects with impaired fasting glucose. Platelets. 2006;17:67-9.
- [Google Scholar]
- Serotonin Antagonism Improves Platelet Inhibition in Clopidogrel Low-Responders after Coronary Stent Placement: An In vitro Pilot Study?. Available from: http://www.journalsplos.org/plosone/article?id=101371/journal.pone.0032656
- Assessment of platelet activation indices using the ADVIATM 120 amongst ‘high-risk’ patients with hypertension. Ann Med. 2007;39:72-8.
- [Google Scholar]
- Increased platelet cholesterol and decreased percentage volume of platelets as a secondary risk factor for coronary artery disease. Pathophysiol Haemost Thromb. 2007;36:45-51.
- [Google Scholar]
- Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46:3-10.
- [Google Scholar]
- Association of mean platelet volume with the degree of retinopathy in patients with diabetes mellitus. Eur J Gen Med. 2009;6:99-102.
- [Google Scholar]





