Translate this page into:
Concurrent evaluation of microscopic observation of drug susceptibility assay for pulmonary and extrapulmonary tuberculosis
Address for correspondence: Dr. Sonali Sudhir Zadbuke, Department of Microbiology, Jawaharlal Nehru Medical College, Sawangi (Meghe), Wardha, Maharashtra, India. E-mail: drsonali07@gmail.com
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
BACKGROUND:
Methods for detection and drug susceptibility of tuberculosis (TB) with solid media are inexpensive but slow and laborious. Rapid methods to diagnose TB and multidrug-resistant TB (MDR-TB) are a global priority for TB control.
OBJECTIVES:
A study was performed to compare the sensitivity of detection of mycobacterial growth and time of culture positivity by microscopic observation of drug susceptibility (MODS) assay with that of Lowenstein–Jensen (LJ) culture in pulmonary and extrapulmonary TB and to evaluate the concordance of the susceptibilities to isoniazid (INH) and rifampicin (RIF) by MODS and proportion method on LJ.
MATERIALS AND METHODS:
A prospective, laboratory-based study was conducted on a total of 300 samples from suspected cases of pulmonary and extrapulmonary TB. Samples were inoculated on LJ medium as per the standard guidelines and MODS assay was performed.
RESULTS:
Sensitivity of MODS assay was 80% and 83.3% and specificity was 92.9% and 83.3% for pulmonary and extrapulmonary samples, respectively. Difference between mean time to detection of Mycobacterium TB (MTB) by LJ medium and MODS was statistically significant, with MODS being faster. drug susceptibility testing (DST) by MODS when compared to economic variant of proportion method was 87.87% for RIF, 90.9% for INH, and 96.96% for MDR-TB detection.
CONCLUSION:
MODS assay provides rapid, safe, and sensitive detection of TB faster than the existing gold standard. It is extremely promising in effectively diagnosing MDR-TB.
Keywords
Extrapulmonary tuberculosis
microscopic observation of drug susceptibility assay
pulmonary tuberculosis
Introduction
Tuberculosis (TB) has the dubious distinction of being the most persistent scourge of humankind.[1] The WHO estimates that now 9 million people developed TB in 2013 and 1.5 million died.[2] Multidrug-resistant TB (MDR-TB) is resistance to the first-line anti-TB drugs – rifampicin (RIF) and isoniazid (INH). MDR-TB accounts for higher mortality and is far more expensive to treat than TB sensitive to the first-line drugs. Lack of diagnostic capacity has been a crucial barrier preventing an effective response to the challenges of HIV-associated and drug-resistant TB, with only 7% of the estimated global burden of MDR-TB cases being detected. Therefore, the expanded capacity to diagnose TB and MDR-TB is a global priority for TB control.[3] Conventional culture and drug susceptibility testing (DST) methods require prolonged periods, i.e., up to 4–8 weeks to confirm mycobacterial growth and detect drug resistance, during which time patients may be inappropriately treated, drug-resistant strains may continue to spread, and amplification of resistance may occur. Molecular methods are expensive and require well-trained personnel. In addition, not all mutations concurring resistance to anti-TB drugs are known. Microscopic observation of drug susceptibility testing (MODS) can be a suitable test in such settings. MODS is a liquid culture-based assay that detects Mycobacterium TB (MTB) and assays INH and RIF susceptibility directly from the sample. It is based on three important properties of MTB: (i) It grows faster in liquid as compared to solid medium, (ii) in liquid medium, MTB grows in a visually characteristic manner (tangles, cording) which can be observed under microscope long before the naked eye can visualize colonies on the solid agar, and (iii) DST from clinical samples is possible by adding anti-TB drugs into broth cultures at the very beginning. Moreover, sample preparation for MODS is similar to that required for preparation of a sputum sample for smear and culture.[4]
Majority of the studies on MODS assay have been done on pulmonary specimens. Therefore, we conducted a study to compare the sensitivity and specificity of detection of mycobacterial growth of MODS assay with that of Lowenstein–Jensen (LJ) culture in both pulmonary and extrapulmonary samples. We also compared the time of culture positivity by both MODS and LJ and evaluated the concordance of the susceptibilities to INH and RIF by MODS and proportion method on LJ.
Materials and Methods
A prospective, laboratory-based study was conducted in a tertiary care hospital in Mumbai after approval from the Institutional Ethics Committee. The study was conducted over a period of 1 year from January to December 2011. A total of 300 samples were collected from suspected cases of TB. Samples collected included early morning sputum, fine needle aspirates, body fluids, pus, and urine. The work was carried out in Class II biosafety cabinet, and biosafety level 2 practices were followed.
Microscopic observation of drug susceptibility assay
All samples were digested and decontaminated using N-acetyl-L-cysteine-NaOH method as per the standard protocol.[56]
The MODS assay was performed as described in the standard operating procedure.[56]
To each 4.5 ml of Middlebrook 7H9 broth, 0.5 ml oleic acid-albumin-dextrose-catalase (OADC) (10%) and 0.1 ml polymyxin b, amphotericin b, nalidixic acid, trimethoprim, and azlocillin (PANTA) were added adding to total of 5.1 ml of broth. The decontaminated sample sediment was resuspended in Middlebrook 7H9 broth with OADC and PANTA supplement for MODS assay.
0.4 mg/ml of INH stock solution was prepared using sterile distilled water as diluents and 1 mg/ml of RIF stock solution was prepared using dimethyl sulfoxide as diluent. These were stored in 120 µl aliquots in microcentrifuge tubes at −20°C. Working solutions of each drug were prepared on the day of use from stock solution. Dilutions were made using Middlebrook 7H9 with 10% OADC. Using a tuberculin syringe, 100 µl (400 µg/ml) of thawed INH stock solution was added to 900 µl of 7H9-OADC which yielded INH stock 2, 40 µg/ml. 100 µl of INH stock 2 was added to another 900 µl of 7H9-OADC yielding 4 µg/ml INH working solution. Similarly, using a tuberculin syringe, 100 µl (1 mg/ml) of thawed RIF stock solution was added to 900 µl of 7H9-OADC which yielded RIF stock 2, 100 µg/ml. 100 µl of RIF stock 2 was added to another 900 µl of 7H9-OADC yielding 10 µg/ml RIF working solution.
Microscopic observation of drug susceptibility plate inoculation
For each sample, four wells in a column of a sterile 24-well tissue culture plate were used (2 drug-free controls and 2 with drugs). Using 7H9-OADC-PANTA (from the tube containing 5.1 ml), the decontaminated sample pellet was resuspended in a total volume of 2 ml in the centrifuge tube and mixed well. To each of 4 wells, 900 µl of sample-broth mixture was added. Remaining 1.7 ml of decontaminated sample was stored as “backup,” in case repeat culture was needed. In the first two wells, 100 µl of supplemented Middlebrook 7H9 broth was added. These two wells acted as control. In the third well, 100 µl of INH (4 µg/ml) was added to obtain the final concentration of 0.4 µg/ml. In the fourth well, 100 µl of RIF (10 µg/ml) was added to obtain the final concentration of 1 µg/ml. On each plate, lane 3 was used as negative control. Only supplemented Middlebrook 7H9 broth, 100 µl of INH and RIF were used without the addition of any sample. Any growth in this lane indicated cross-contamination. On each processing day, two positive controls were run. H37RV was used as a sensitive control and one known MDR strain was used as resistant control. Tissue culture plates were sealed by cello tape and were incubated at 37°C.
Microscopic observation of drug susceptibility plate reading
The plates were examined with cello tape seal daily under an inverted microscope starting from day 1. MTB growth was identified by characteristic cord formation in drug-free wells. Bacterial or fungal contamination was identified by clouding of media within 5 days of inoculation. A negative MODS culture was discarded after 21 days. Reading of the plates was done from day 1 under the inverted light microscope. If results were negative on day 5, reading drug-free wells was done daily or on alternate days according to the laboratory workload until >2 CFU (colony forming units) was observed in each of the two wells. When positive results were observed, the INH- and RIF-containing wells were read on the same day. If no growth was observed by day 15, reading on day 18 and day 21 were repeated. If results were still negative on day 21, the final result was reported as negative.
Microscopic observation of drug susceptibility interpretation
Growth in control wells, without growth in drug-containing wells, was recorded as sensitive. If growth was detected in both control and drug-containing wells, it was reported as resistant. Sensitivity and specificity of MODS assay were calculated using results of culture method as the gold standard.
Quality Control: With each batch of freshly prepared media, the standard strain of MTB H37Rv (ATCC 27294) was tested with the drug-free as well as the drug-containing media.
Culture on Lowenstein–Jensen media: Two loopful of decontaminated sediments was inoculated on LJ medium and incubated aerobically at 37°C. All cultures were read daily for the first week for contamination and rapidly growing mycobacterial species and then weekly thereafter till growth was detected or 12 weeks, whichever was later[7] as in our experience many MTB isolates grow in the tenth or eleventh week.
Any growth observed on the LJ medium was confirmed as acid-fast bacilli by ZN staining and identified as MTB or MOTT using phenotypic characteristics such as rate of growth, pigment production, niacin accumulation test, nitrate reduction test, and semi-quantitative catalase test.
Any slow-growing acid-fast isolate which was buff colored, niacin and nitrate positive, and semi-quantitative catalase negative was identified as MTB. Isolates identified as MTB were stored at −70°C. They were subcultured on LJ medium to obtain fresh cultures before use. Isolates were subjected to susceptibility testing using two important primary drugs (INH and RIF) by economic variant of proportion method using LJ medium.
Drug susceptibility testing by economic variant of proportion agar method: 48 clinical isolates of MTB were tested for drug susceptibility by proportion method.
LJ medium was prepared as per the WHO guidelines. The drug-containing media and inoculums were prepared as per the protocol.
Quality control
With each batch of freshly prepared media, the standard strain of MTB H37Rv (ATCC 27294) was tested with the drug-free as well as the drug-containing media.
Incubation and reading
The inoculated slopes were placed at an angle and incubated at 37°C. They were examined for contamination after 1 week. The results were read for the first time on the 28th day. If the strain was susceptible, then it was incubated for the second reading on the 42nd day as the final reading.
Growth was recorded as 1–99 colonies: The actual number of colonies:
-
++: More than 100 colonies
-
+++: Confluent growth.
Interpretation of the test
Any strain with 1% (the critical proportion) of bacilli resistant to any of the drugs was classified as resistant to that drug.
Statistical analysis
Unpaired t-test was applied to compare time to detection by LJ medium and MODS assay. P < 0.05 was considered statistically significant. Data were analyzed using SPSS software version 16.0. IBM Corp., Armonk, New York, United States.
Results
A total of 300 consecutive samples of suspected TB among adult patients received in mycobacteriology laboratory were included in the study. Out of 300 samples, 7 grew rapidly growing mycobacteria, 10 showed contamination on LJ medium, 12 samples showed contamination on MODS, and 9 grew contaminants on both. Therefore, these 38 samples were excluded from the final analysis [Figure 1].
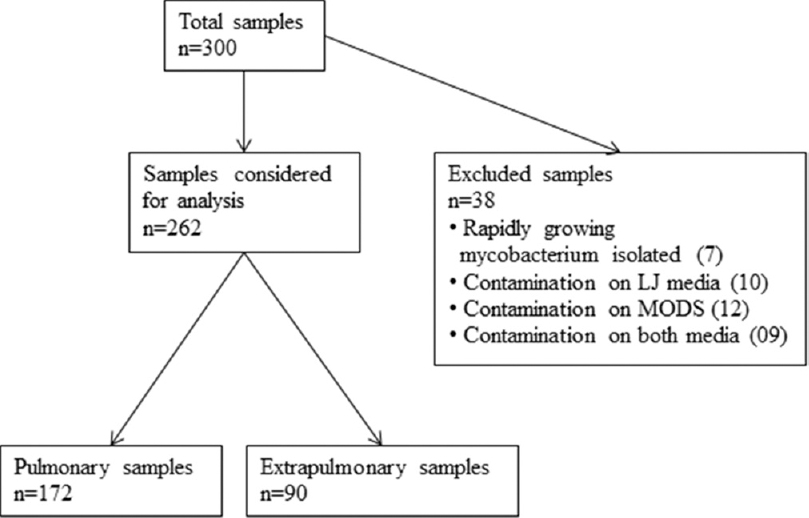
- Chart for workflow of specimens in the study
Of the 262 samples analyzed, 172 were sputum samples while 90 were from extrapulmonary TB which included pus, fine needle aspiration cytology (FNAC), pleural fluids, and urine and other body fluids.
Table 1 shows the comparison of MODS assay with conventional culture on LJ media for pulmonary samples and extrapulmonary samples. The sensitivity and specificity of MODS culture as compared to LJ culture were 80% and 92.9%, respectively, for pulmonary samples and for extrapulmonary samples both were 83.33%. The sensitivity and specificity of MODS culture as compared to LJ culture were 81.25% and 89.71%, respectively, for total samples. Sensitivity values for pulmonary and extrapulmonary samples were comparable while specificity was on the lower side for extrapulmonary samples.
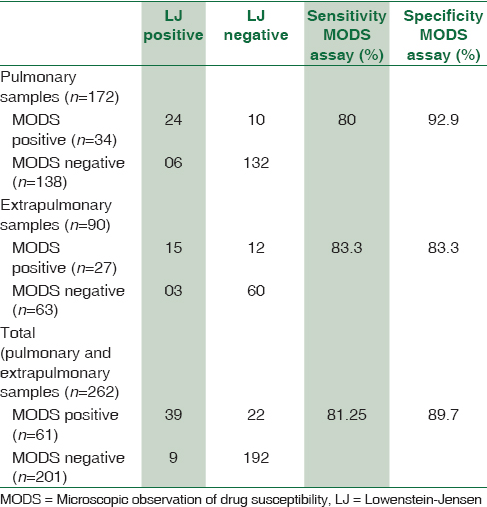
Mean time to detection of growth by MODS assay was around 10 ± 4.44 days as compared to 31 ± 12.6 days on LJ medium. This difference was statistically significant (P < 0.001) on applying unpaired t-test.
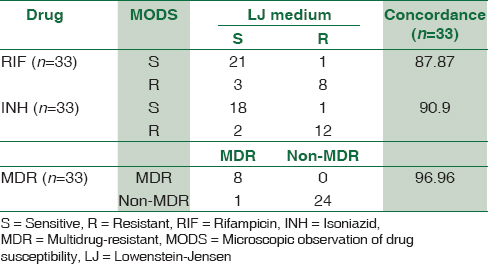
Drug susceptibility by both methods was performed on 33 isolates. Concordance of DST between the MODS assay and the economic variant of proportion method for RIF, INH, and MDR-TB is shown in Table 2. The concordance of DST by MODS for RIF, INH, and MDR-TB when compared to the economic variant of proportion method was 87.87%, 90.0%, and 96.96%, respectively.
Discussion
TB and MDR-TB prevalent areas are typically resource-poor, prohibiting the use of expensive sputum culture and drug susceptibility testing (DST) methods. Diagnosis in these areas depends on sputum microscopy which misses half of the cases and DST is only performed after therapy fails. DST methods with solid media are inexpensive but slow and laborious. Liquid-automated commercial systems are rapid but require heavy, expensive equipment, have high running costs, and are technically complex. Molecular methods are expensive and require well-trained personnel. In addition, not all mutations concurring resistance to anti-TB drugs are known. In such a situation, the MODS assay is a simple and rapid method to detect both the presence of the MTB and susceptibility of the organism to INH and RIF.
Sensitivity for detection of MTB by MODS in pulmonary, extrapulmonary, and total samples in the present study was 80%, 83.33%, and 81.25%, respectively, when compared to LJ medium [Table 1]. In literature, very high sensitivity of detection of 97.8% has been reported for this assay for pulmonary specimens.[8] Michael et al. in their study from India have reported a sensitivity of 78.9% for MODS assay when compared to reference solid or liquid culture.[9] Shah et al.[10] in their study from South Africa have reported sensitivity of 85% which is similar to our study. The authors mentioned that a lower sensitivity may be caused by the number or type of sputum samples they collected from each patient (single spot sample), or sample storage, processing, or splitting, which may have significantly reduced the bacillary volume in each inoculum. Sensitivity may be improved slightly by performing multiple MODS assays, similar to other evaluations of rapid molecular tests on sputum.[10] Mashta et al. in a recent study from India have reported a sensitivity of 48% in smear-positive and 38% in smear-negative cases. The reason given was perhaps there is flawed generalization that all pathogenic MTB form cords.[11]
One point of explanation of modest sensitivity in the present study is that we included consecutive samples received in our laboratory from suspected adult TB cases irrespective of smear findings. Second, this was a pilot study in our institute being performed for the first time. Repeated studies with supervised training can improve the detection of MTB by MODS. Attorri et al. had demonstrated cording in 64% of MTB growing from liquid cultures (BACTEC). However, they demonstrated that with training, their sensitivity of detecting true cording in MTB in liquid culture increased to 79% and reemphasized that without training and experience, serpentine cords can be missed or confused with pseudocords.[12]
The present study shows almost comparable sensitivities for pulmonary and extrapulmonary samples as 80% and 83.33%, respectively [Table 1]. Tovar et al. have reported sensitivity of 66% in pleural biopsy samples and 14% in pleural fluid samples.[13] Caws et al. have reported sensitivity of 80.4% in cerebrospinal fluid (CSF) samples.[14] We have found comparatively higher sensitivity of 83.3% in extrapulmonary samples. Our samples consisted more of pus and FNACs which might have accounted for higher sensitivities as pleural fluid and CSF samples are paucibacillary and give less culture yield.[1516]
Dang et al. had included pulmonary and extrapulmonary samples such as CSF and pleural fluid in their study and had found a sensitivity of 88.6% when analyzed by patient against microbiological confirmation.[17] The present study also had an overall sensitivity of 81.25%.
Specificity of detecting MTB by MODS in the present study was 92.9%, 83.33%, and 89.71%, respectively, for pulmonary, extrapulmonary, and total samples [Table 1]. In literature, higher specificities of around 96–100% have been reported which include 99.6% by Moore et al., 100% by Limaye et al., 96.7% by Michael et al., and 94.4% by Mayra et al.[891819] Lazarus et al. have reported specificity of 89.39% for MODS done on pulmonary samples which is similar to the present study.[20] Specificity for pulmonary samples in the present study is comparable to others whereas for extrapulmonary samples, it is on the lower side. Specificity has recently been improved by revising MODS platform to include a well with para-n-nitrobenzoic acid (PNB). The absence of growth in PNB combined with cord formation in drug-free well will be specific for MTB.[10] However, as we did not have an automated liquid culture medium such as MGIT or BACTEC available due to cost constraint, we were unable to explain cases which were MODS positive but LJ negative. This was a limitation of our study.
In the present study, MODS took a mean time of 10 ± 4.4 days to detect MTB while the same was 31 ± 12.5 days on LJ medium. This difference was statistically significant with P < 0.001. The less turnaround time using MODS is important for better and effective patient care since MODS helps detect MTB specifically based on its growth characteristic in liquid medium. Mean time to detection was comparable with other studies.[48181921]
Concordance of DST by MODS when compared to economic variant of proportion method was 87.87% for RIF, 90.9% for INH, and 96.96% for MDR-TB detection [Table 1]. Lazarus et al. have also reported concordance of DST by MODS to RIF and INH when compared to the proportion method as 91.5% and 90.8%, respectively.[20] Limaye et al. reported 100% agreement between MODS and proportion agar method for MDR-TB detection.[18] Our results were comparable to these studies.
In MODS assay, DST results were available on the same day as detection, i.e., around 10 days whereas additional 42 days were required for DST results by economic variant of proportion method. Given the high mortality from TB and MDR-TB and the prolonged opportunity for TB transmission prior to diagnosis, MODS assay can be of great potential for detection of TB and MDR-TB in resource-limited settings. This will contribute to patient management outcomes. An added benefit is the isolation of MTB from MODS drug-free well for genotyping and second-line DST. Definitely MODS augments smear microscopy by providing rapid DST. The minimum equipment required is a biological safety cabinet, a biosafety centrifuge, vortex, incubator, and an inverted light microscope. After inoculation with decontaminated sample, the MODS plates are permanently sealed; thus, spillage of the mycobacterial soup cannot occur. Furthermore, no secondary subculture is needed as is it is a direct DST. Hence, there is zero potential for aerosolization or accident. All consumables and reagents are available from the standard laboratory suppliers. Training requirements are moderate. It is useful for trainees to gain an appreciation of the range and variety of appearances of the pattern of growth.
We acknowledge that there are a few limitations in the present study. This was a laboratory-based study and the results were not used for patient management. Hence, we could not evaluate the impact of our findings on TB or MDR-TB. No third method such as automated liquid culture (MGIT or BACTEC) was available for comparison to explain cases which were MODS positive but LJ negative.
Conclusion
Thus, MODS assay provides rapid, safe, and sensitive detection of TB faster than the existing gold standards. It is extremely promising and has the potential to effectively diagnose MDR-TB.
Financial support and sponsorship
The financial support was provided by Research Society, Topiwala National Medical College, and BYL Nair Charitable Hospital.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We acknowledge Research Society, Topiwala National Medical College, and BYL Nair Charitable Hospital.
References
- Tuberculosis: epidemiology and control. Tuberculosis: epidemiology and control. 2002
- [Google Scholar]
- WHO. Global Tuberculosis Report 2014. Geneva: WHO; 2014. p. :1-289.
- TB Diagnostics and Laboratory Services. Available from: http://www.who.int/tb/dots/lab.pdf
- Microscopic observation drug susceptibility assay, a rapid, reliable diagnostic test for multidrug-resistant tuberculosis suitable for use in resource-poor settings. J Clin Microbiol. 2004;42:4432-7.
- [Google Scholar]
- MODS: A user guide. Lima Peru: Universidad Peruana Cayetano Heredia; 2008.
- Rapid identification and drug susceptibility testing of Mycobacterium tuberculosis: Standard operating procedure for non-commercial assays: Part 1: Microscopic observation drug susceptibility assay v2.4. J Lab Physicians. 2012;4:101-11.
- [Google Scholar]
- World Health Organization. Laboratory services in tuberculosis control. Part III. Culture. Geneva, Switzerland: WHO; 1998.
- Microscopic-observation drug-susceptibility assay for the diagnosis of TB. N Engl J Med. 2006;355:1539-50.
- [Google Scholar]
- Diagnostic accuracy of the microscopic observation drug susceptibility assay: A pilot study from India. Int J Tuberc Lung Dis. 2010;14:482-8.
- [Google Scholar]
- Rapid diagnosis of tuberculosis and multidrug resistance by the microscopic-observation drug-susceptibility assay. Am J Respir Crit Care Med. 2011;183:1427-33.
- [Google Scholar]
- Diagnosis of tuberculosis: The experience at a specialized diagnostic laboratory. J Negat Results Biomed. 2011;10:16.
- [Google Scholar]
- Assessment of morphology for rapid presumptive identification of Mycobacterium tuberculosis and Mycobacterium kansasii. J Clin Microbiol. 2000;38:1426-9.
- [Google Scholar]
- Improved diagnosis of pleural tuberculosis using the microscopic- observation drug-susceptibility technique. Clin Infect Dis. 2008;46:909-12.
- [Google Scholar]
- Evaluation of the MODS culture technique for the diagnosis of tuberculous meningitis. PLoS One. 2007;2:e1173.
- [Google Scholar]
- Improving the bacteriological diagnosis of tuberculous meningitis. J Clin Microbiol. 2004;42:378-9.
- [Google Scholar]
- Microscopic observation drug susceptibility assay (MODS) for early diagnosis of tuberculosis in children. PLoS One. 2009;4:e8341.
- [Google Scholar]
- Utility of microscopic observation of drug susceptibility (MODS) assay for Mycobacterium tuberculosis in resource constrained settings. Indian J Tuberc. 2010;57:207-12.
- [Google Scholar]
- Clinical evaluation of the microscopic-observation drug-susceptibility assay for detection of tuberculosis. Clin Infect Dis. 2007;44:674-80.
- [Google Scholar]
- Evaluation of the microscopic observational drug susceptibility assay for rapid and efficient diagnosis of multi-drug resistant tuberculosis. Indian J Med Microbiol. 2012;30:64-8.
- [Google Scholar]
- Evaluation of microscopic observation drug susceptibility assay for detection of multidrug-resistant Mycobacterium tuberculosis. J Clin Microbiol. 2007;45:1093-7.
- [Google Scholar]





