Translate this page into:
An atypical presentation of alveolar soft part sarcoma of tongue
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
Alveolar soft part sarcoma (ASPS) is a rare and aggressive tumor in adolescents and young adults with uncertain histogenesis. It comprises <1% of all soft tissue sarcomas with a female predominance. ASPS commonly involves the upper and lower extremities and is uncommonly seen in the head and neck region.[1] It usually affects young adults and adolescents between 15 and 35 years of age. Tumors occurring in infancy and childhood have a predilection for head and neck region, especially orbit and tongue.[2] There is slight female preponderance before the age of 30 and slight male preponderance over 30 years of age.
A 35-year-old male presented with mass in the right lateral border of tongue [Figure 1], associated with slowly progressive dysphagia for solids and a single episode of oral bleeding. The lesion developed as a 0.5 cm nodule and progressed to its present size involving right lateral half of the tongue over a period of 3 months. His oropharyngeal examination revealed soft fleshy mass of 2 cm × 2 cm involving right lateral border of tongue. Difficulty in tongue movement was also noted. Contrast-enhanced computed tomography showed an irregularly enhancing lesion in the right lateral aspect of tongue measuring 3.3 cm × 2.1 cm reaching up to medial aspect of left hemitongue and inferiorly up to sublingual space on the right side. Enlarged lymph nodes were also noted in bilateral submandibular and upper deep cervical regions.
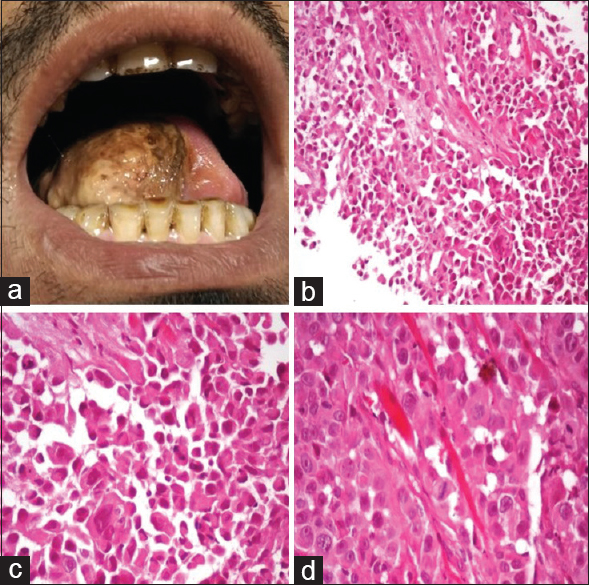
- (a) Soft fleshy mass involving right hemitongue. (b) Cells arranged in pseudoalveolar pattern (H and E, ×100). (c and d) Cells with abundant granular eosinophilic cytoplasm, large nuclei with open chromatin and prominent nucleoli (H and E, ×200 and ×400)
An incisional biopsy was performed [Figure 1] which revealed tumor in subepithelium composed of epithelioid cells arranged in alveolar and solid pattern with intervening thin-walled blood vessels. The cells had a moderate amount of cytoplasm, round nuclei, and prominent eosinophilic nucleoli and showed increased mitotic activity. Immunohistochemical studies and special stains were performed to confirm the diagnosis. The tumor was positive for neuron-specific enolase (NSE) and vimentin and negative for desmin, HMB-45, and cytokeratin (CK). Periodic acid–Schiff-reaction with diastase digestion highlighted intracytoplasmic needle-shaped crystals consistent with the diagnosis of ASPS [Figure 2]. Computerized tomographic examination of the chest and abdomen was performed, and metastasis to lung and liver ruled out. The patient was referred to an oncology center for oncosurgery and further management.
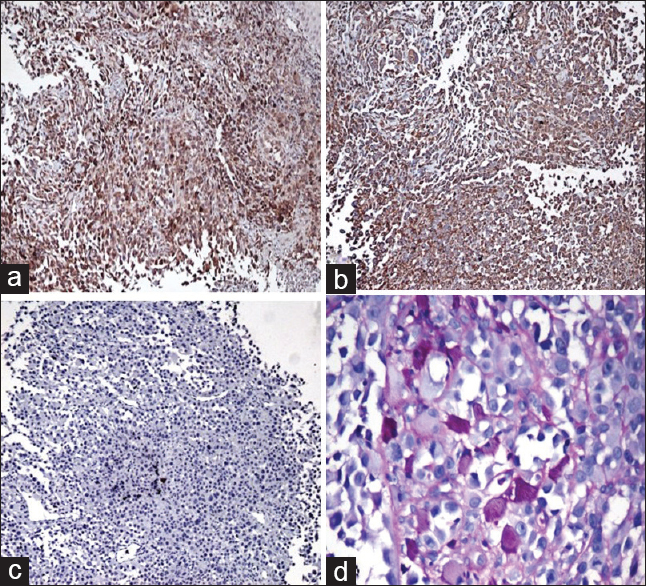
- Immunohistochemical findings. (a) Diffuse cytoplasmic staining with neuron-specific enolase. (b) Cytoplasmic staining with vimentin. (c) Negative staining with cytokeratin. (d) Periodic acid–Schiff-positive, diastase-resistant crystals (PAS, ×200)
The first case of ASPS was described in 1952.[2] ASPS of tongue is usually a slow-growing tumor; however, in our case, patient presented with a rapidly growing lesion over a period of 3 months, which has not been described previously. The clinicopathologic characters of oral ASPS differ from that of extremities. Oral ASPS shows higher female predominance than extremity ASPS. Lingual ASPS is usually smaller in size. Smaller size and early diagnosis account for better prognosis of oral ASPS.[3]
ASPS is classified under tumors of uncertain differentiation in 2002 WHO classification of soft tissue tumors. Earlier ASPS was considered to be of neural origin, but recent immunohistochemical studies have proved it to be of uncertain differentiation.[4] Twenty-five percent cases demonstrate NSE or S-100 positivity.[5] CK, epithelial membrane antigen, and neurofilament have been consistently negative. ASPS is usually slow growing and deceptively appears histologically benign with no atypical mitosis or nuclear pleomorphism. However, in our case, the mitotic activity was considerably high with the presence of atypical mitosis. The tumor spread is primarily hematogenous with lymphatic involvement being extremely rare being reported in 7% cases of head and neck ASPS.[1] The differential diagnostic possibilities include granular cell tumor, perivascular epithelioid cell tumors, clear cell sarcoma, extrarenal rhabdoid tumor, paraganglioma, and alveolar rhabdomyosarcoma.[2] The pathognomonic histologic feature of ASPS[2] is the presence of granules and rhomboid/rod-shaped crystalline intracytoplasmic inclusions reported in 25%–100% cases.[2] Periodic acid–Schiff-positive, diastase-resistant granules may represent precursors to rod-shaped crystals.
Complete surgical excision is the mainstay of treatment. Role of adjuvant therapy remains controversial, but radiotherapy/chemotherapy may be given in cases of inadequate surgical excision.[26] The prognostic parameters of ASPS include age at diagnosis, tumor size, and presence of metastasis. The clinical features and microscopic appearance of ASPS are variable and may develop unusual features as in our case. Lingual ASPS has a good prognosis and long tumor-free survival if diagnosed and treated early. A thorough histologic and radiologic examination becomes the basis of reliable diagnosis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Alveolar soft-part sarcoma of the head and neck: Clinical and imaging features in five cases. AJNR Am J Neuroradiol. 2005;26:1331-5.
- [Google Scholar]
- Oral alveolar soft part sarcoma in childhood and adolescence: Report of two cases and review of literature. Head Neck Pathol. 2013;7:40-9.
- [Google Scholar]
- Rare tumor of the tongue in a child: Alveolar soft part sarcoma. Pediatr Dev Pathol. 2009;12:147-51.
- [Google Scholar]
- Molecular genetic, cytogenetic, and immunohistochemical characterization of alveolar soft-part sarcoma. Implications for cell of origin. Cancer. 1992;70:2444-50.
- [Google Scholar]
- Alveolar soft part sarcoma of tongue base – A rare presentation of a rare tumor. Indian J Otolaryngol Head Neck Surg. 2007;59:393-5.
- [Google Scholar]
- Angiogenesis-promoting gene patterns in alveolar soft part sarcoma. Clin Cancer Res. 2007;13:7314-21.
- [Google Scholar]




