Translate this page into:
Study of vancomycin and high-level aminoglycoside-resistant Enterococcus species and evaluation of a rapid spot test for enterococci from Central Referral Hospital, Sikkim, India
Address for correspondence: Dr. Tsering Yangzom, Department of Microbiology, Sikkim Manipal Institute of Medical Sciences, Sikkim Manipal University, 5th Mile, Tadong, Sikkim, India. E-mail: mitseyang@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
BACKGROUND:
Enterococcus is an important pathogen, and with its emergence of resistance to multiple antimicrobials, the management of infection is becoming increasingly difficult.
AIM:
The aim of the study is to determine the prevalence, antibiotic resistance, and risk factors associated with enterococcal infection or colonization.
MATERIALS AND METHODS:
In this prospective study, samples from inpatients were screened for resistant enterococci. Antibiotic susceptibility testing was performed using the disc diffusion method and minimum inhibitory concentration by the agar dilution method. A modification of a test tube method of sodium chloride-esculin hydrolysis to a spot test was evaluated for its rapidity and reliability in the presumptive diagnosis of enterococci.
STATISTICAL ANALYSIS USED:
Fisher's exact test was used for continuous (Student's t-test) and categorical variables. Multivariate analysis was performed with logistic regression using IBM SPSS 20.0 software (Armonk, NY, USA).
RESULTS:
Enterococcus species were isolated from 182 samples: Enterococcus faecalis (68.7%), Enterococcus faecium (20.9%), Enterococcus gallinarum (6%), and Enterococcus durans (4.4%). Maximum resistance was to ciprofloxacin (59.3%) and least to linezolid (0.5%). The isolation rate of vancomycin-resistant enterococci (VRE) was 13.7%; 30.2% and 20.9% were of high-level gentamicin and streptomycin, respectively. All 182 Enterococcus species gave positive results within 30–60 min by the rapid spot test.
CONCLUSIONS:
Overall, high-level aminoglycoside resistance (HLAR) was observed more than glycopeptide resistance. Surveillance strategies need to be upgraded and implemented in order to prevent the emergence and further spread of not only VRE but also HLAR enterococci in the hospital. The spot test gave reliable and rapid results in presumptive identification of enterococci.
Keywords
Enterococcus
high-level aminoglycoside resistance
rapid spot test
vancomycin-resistant enterococci
Introduction
Enterococcus species which forms part of the commensal flora of gastrointestinal tracts of humans, avian and veterinary origins are also known to be pathogenic organisms of medical importance.[123] They have the ability to acquire resistance (plasmid mediated) and are intrinsically resistant to commonly used antibiotics (such as clindamycin, cephalosporins, low-level aminoglycoside, and co-trimoxazole). Hence, enterococci are often considered as a “pathogen in training.”[245]
Enterococcus is a ubiquitous organism; often, the ecology depends on how actively enterococci are distributed and controlled.[26] In Europe, the reservoirs of resistant enterococci, particularly vancomycin-resistant enterococci (VRE), shifted from a veterinary to a community-acquired ecology whereas, in the USA, the source is often the nursing home/hospital environment.[2] However, in India, the source of resilient enterococci is not well-defined. Similarly, in this region, there is a paucity of information on the prevalence of enterococci in hospitals as well as community-acquired settings. The aim of this study was to actively screen and identify the isolated Enterococcus species and determine its antimicrobial resistance pattern at a tertiary hospital in East Sikkim. The objective of this study was to detect glycopeptide-resistant and high-level aminoglycoside-resistant (HLAR) enterococci among patients and analyze the risk factors associated with infection or colonization. In addition, we sought to assess an in-house rapid spot test for the presumptive identification of enterococci (modification of a test first described by Qadri et al.) and its ability to distinguish enterococci from similar streptococci (catalase-negative Gram-positive cocci).[7]
Materials and Methods
Study design
This is a cross-sectional study (prevalence study) conducted in the Department of Microbiology, Central Referral Hospital (CRH), East Sikkim, India, from November 2016 to April 2018. The study was reviewed and approved by the Institution Ethics Committee.
Study population
All patients admitted to CRH during the study period whose samples were sent to the Microbiology Department for other investigations were analyzed.
Methodology
The patients enrolled in the study were those (a) with fever >38°C, (b) without fever, and (c) who developed a fever during their hospital stay. The diagnosis of infection was based on the guidelines formulated by the Centers for Disease Control and Prevention.[8] The diagnosis of enterococcal infection was established when at least three criteria were met: (a) positive culture, (b) clinical signs and symptoms of fever >38°C, (c) >10 leukocytes per high-power field in a preliminary Gram staining report, and/or (d) white blood cell >12000 or <4000 cells/mm3.
Nosocomial infection was defined as an infection occurring in patients with >48 h of hospital stay or infection in those with a history of recent hospitalization (2 weeks). Patients with a positive culture without signs and symptoms of infection were deemed to be colonizers.
Detailed history pertaining to demography, immune status (comorbidities and immunosuppressive therapy), antibiotic therapy, location of patient, duration of stay, invasive procedures (such as Foley's catheter and central venous catheters), or surgery and history of recent hospitalization or intensive care unit (ICU) stay (≤30 days ago) were recorded for samples positive for enterococci. Length of hospital stay and assessment of clinical outcomes were recorded from the day of admission till discharge or death. Primary and secondary bloodstream infections (BSI) were defined accordingly.[8] To overcome repetitive sampling, >1 Enterococcus isolates of the same patient but from different sites and on multiple occasions were considered as a single sample, i.e., only the first isolate was considered.
Microbiological sample processing
All samples from inpatient wards, sent for culture to the Department of Microbiology, were selected according to the criteria proposed in the study methodology. For blood culture, paired samples were inoculated in blood culture bottles (BacT/Alert FA Plus for adults and PF Plus for pediatric patients) and cultured in BacT/Alert systems (bioMérieux, France) for a period of at least 7 days. For urine culture, colony counts of ≥105 colony-forming units per ml (CFU/ml) were evaluated. Selected samples were cultured in conventional media and screened for presumptive vancomycin resistance on a VRE screen agar and prepared using bile esculin azide agar supplemented with 6% (6 μg/ml) vancomycin (HiMedia Laboratories, Mumbai, India).[4910] Identification of Enterococcus species by conventional biochemical reactions (growth on potassium tellurite agar, 6.5% sodium chloride (NaCl) broth, 1% sugar fermentation tests, esculin hydrolysis, and arginine hydrolysis) and results interpreted by the identification scheme proposed by Facklam and Collins.[2311]
Antibiotic susceptibility testing
Antimicrobials against enterococci were tested by the Kirby–Bauer disc diffusion method with antibiotic discs (HiMedia Laboratories, Mumbai, India) for ampicillin (10 μg), ciprofloxacin (5 μg), vancomycin (30 μg), teicoplanin (30 μg), high-level gentamicin (HLG, 120 μg), high-level streptomycin (HLS, 300 μg), and linezolid (30 μg). Nitrofurantoin (300 μg) was tested for urine isolates only. Minimum inhibitory concentration (MIC) for glycopeptides was determined by the agar dilution method on brain–heart infusion agar (MIC: 0.125–256 μg/ml). Isolated organisms on 6% VRE screen agar were tested for their breakpoint MIC values by the vancomycin agar dilution method. Test for detection of HLAR by the agar dilution method was done using gentamicin (500 μg/ml) and streptomycin (2000 μg/ml). In all the agar dilution methods, the presence of >1 colony indicated resistance. All the MIC determinations were performed as per the guidelines set by the Clinical and Laboratory Standards Institute (CLSI-M100S, 26th edition).[10] The reference strains of Enterococcus faecalis ATCC® 29212™ (sensitive) and ATCC® 51299™ (resistant) were used as control while testing the antimicrobial susceptibility testing (AST) and MIC values.[10] VRE and high-level gentamicin resistance (HLGR) findings by agar dilutions were also consistent with VITEK 2 systems reporting, using VITEK 2 GP and VITEK 2 AST-P628 cards (bioMérieux, France). VITEK 2 AST-P628 cards do not report HLS findings. Therefore, a comparison with this card could not be ascertained.
Sodium chloride-esculin hydrolysis rapid spot test
The medium was prepared as per the formulation of Qadri et al. who described it as a tube test method, but an impregnated filter paper method is evaluated in this study.[7] The test solution is a 0.2% esculin and 5% NaCl medium, composed of 2 g esculin (HiMedia Laboratories, Mumbai, India), 0.5 g ammonium ferric citrate, 50 g NaCl, 0.4 g K2HPO4, and 0.1 g KH2PO4 in 1000 ml distilled water (pH 5.6 ± 0.2). About 100 ml was prepared at a time. The precipitate that formed when stored at 4°C –6°C went into solution when lightly heated. A 1.8 cm × 1.8 cm of Whatman filter paper (no. 2) was cut to fit a standard microscope. About 0.25 ml of solution was pipetted over the paper, and a colony of Gram-positive catalase-negative cocci, from a 24-h culture in blood agar, was rubbed in the center of the square. The slides were placed in an 11 cm petri dish, atop supporting glass rods, and incubated aerobically at 37°C for at least 30 min. To avoid drying of the paper during incubation, 2–5 ml water was added to the dish. The appearance of black color over the spot-inoculated area indicated a positive response. In this test, Enterococcus species and Streptococcus species were evaluated with the positive control (E. faecalis ATCC® 29212™ or 51299™) and negative control (Staphylococcus aureus). Validation of the spot test was done by comparing it with the growth of Enterococcus species in bile esculin azide (BEA) agar and 6.5% NaCl broth.
Statistical analysis
Continuous values were expressed as mean ± standard deviation (SD) and compared using the Student's t-test. Categorical values were assessed for key variables with the GraphPad software (San Diego, CA, USA) (risk ratio with 95% confidence interval [CI]). Multiple independent variable analysis was performed using binary logistic regression using IBM Corp. SPSS statistics for Windows, version 20.0 (Armonk, NY, USA). Logistic regression was done to ascertain the effects of antibiotic exposure, hospital-related, host-related, and outcome-related factors on the likelihood of acquiring resistant enterococcal infection or colonization. All the tests were two-tailed, and P ≤ 0.05 was considered statistically significant.
Results
During the study period, a total of 3208 selected samples were screened and 182 Enterococcus species (5.7%) were isolated. The overall mean age of the study population was 33.29 ± 18.25 years (SD); 96 (52.7%) were male and 86 (47.3%) female. Four Enterococcus species were isolated and identified as E. faecalis (125, 68.7%), Enterococcus faecium (38, 20.9%), Enterococcus gallinarum (11, 6.04%), and Enterococcus durans (8, 4.4%) [Table 1]. A total of 27.5% (50/182) enterococcal infections were from new admissions (40 – urinary tract infection [UTI] and 10 – pus), 20.9% (38/182) developed nosocomial infection (12 – BSI and 26 UTI), and 51.6% (94/182) were potential colonizers (44 – urine, 3 – pus, and 47 – respiratory secretions).
| Sample (%) | Bacterial isolates | |||
|---|---|---|---|---|
| Enterococcus faecalis (%) | Enterococcus faecium (%) | Enterococcus gallinarum (%) | Enterococcus durans (%) | |
| Urine: 110 (60.4) | 81 (73.6) | 19 (17.3) | 7 (6.4) | 3 (2.7) |
| Blood: 12 (6.6) | 8 (66.7) | 4 (33.3) | 0 (0) | 0 (0) |
| Respiratory fluids: 47 (25.8) | 29 (61.7) | 12 (25.5) | 3 (6.4) | 3 (6.4) |
| Sputum | 20 | 5 | 3 | 2 |
| Throat swab | 6 | 5 | 0 | 0 |
| Endotracheal tube | 3 | 2 | 0 | 1 |
| Pus: 13 (7.1) | 7 (53.8) | 3 (23.1) | 1 (7.7) | 2 (15.4) |
| Total: 182 | 125 (68.7) | 38 (20.9) | 11 (6.04) | 8 (4.4) |
Figures in parentheses are in percentages
Overall, antibiogram of the isolates showed a high resistance to ciprofloxacin at 59.3% (108/182), followed by ampicillin at 53.8% (98/182), HLG at 34.1% (62/182), HLS at 26.9% (49/182), vancomycin at 14.3% (26/182), teicoplanin at 9.9% (18/182), and linezolid at 0.5% (1/182); urine isolates showing resistance to nitrofurantoin were 28.2% (31/110) [Figure 1]. Bar diagram represents the percentage of antimicrobial resistance of the four Enterococcus species.

- Percentage of resistant Enterococcus species by disc diffusion method
By the agar dilution methods, enterococcal resistance to vancomycin was 13.7% (25/182) and for teicoplanin 10.9% (20/182). Resistance to glycopeptides was seen highest in E. faecium, followed by E. faecalis and E. gallinarum. Two isolates of E. faecium had MIC values at 64 and 128 μg/ml, indicating high resistance. Overall, HLGR and high-level streptomycin resistance (HLSR) were 30.2% (55/182) and 20.9% (38/182), respectively, seen highest among strains of E. faecalis, followed by E. faecium, E. gallinarum, and E. durans [Table 2]. HLAR was recorded in all VRE isolates except E. durans. HLGR and HLSR among vancomycin-sensitive (VS) isolates were 22.7% (27/119) and 12.6% (15/119) in VS E. faecalis, 23.1% (6/26) and 11.5% (3/26) in VS E. faecium, respectively, and only 25% (2/80) HLGR in VS E. durans. No HLAR was observed in VS E. gallinarum.
| Isolates | Vancomycin | Teicoplanin | HLG | HLS |
|---|---|---|---|---|
| Enterococcus faecalis (n=125) | 6 (24) | 8 (40) | 33 (60) | 21 (55.3) |
| Enterococcus faecium (n=38) | 12 (48) | 9 (45) | 18 (32.7) | 15 (39.5) |
| Enterococcus gallinarum (n=11) | 7 (28) | 3 (15) | 2 (3.6) | 2 (5.3) |
| Enterococcus durans (n=8) | 0 (0) | 0 (0) | 2 (3.6) | 0 (0) |
| Total | 25 (13.7%) | 20 (10.9%) | 55 (30.2%) | 38 (20.9%) |
Results in parentheses indicate percentage. MIC determined by agar dilution method. HLG=High-level gentamycin (500 µg/ml), HLS=High-level streptomycin (2000 µg/ml). Range of glycopeptide MIC: 0.125 to 256 µg/ml. Breakpoints for vancomycin: [S]: ≥4 µg/ml; [I]: 8-16 µg/ml; [R]: 8-16µg/ml. Breakpoints for teicoplanin: [S]: ≥8 µg/ml; [I]: 16 µg/ml; [R]: 16 µg/ml. MIC=Minimum inhibitory concentration
Rapid sodium chloride-esculin hydrolysis spot test
A total of 230 isolates of enterococci and streptococci were tested [Table 3]. All the 182 enterococcal isolates were positive by the rapid spot test within 30–60 min [Figure 2]. All spot test-positive enterococcal results were positive for growth in BEA agar. Streptococci that did not grow in BEA agar were negative by this rapid spot test. However, two streptococci (with growth on BEA agar) gave positive spot reactions 24 h later. Therefore, to avoid false-positive results, the test was discarded after 90 min.
| Organism | Number of isolates | Number positive | ||||
|---|---|---|---|---|---|---|
| 30 minutes | 1 h | 2 h | 4 h | 24 h | ||
| Enterococcus speciesa | 182 | 74 | 108 | - | - | - |
| Bile esculin-positive streptococcib | 15 | 0 | 0 | 0 | 0 | 2 |
| Streptococcus pyogenes | 8 | 0 | 0 | 0 | 0 | 0 |
| Streptococcus pneumoniae | 5 | 0 | 0 | 0 | 0 | 0 |
| Viridans streptococci | 20 | 0 | 0 | 0 | 0 | 0 |
aAll Enterococcus spp. were positive by both conventional bile-esculin azide agar culture and rapid spot test, bBile esculin-positive streptococci were positive by conventional bile-esculin azide agar culture. Two isolates were positive by the rapid spot test at 4 h and three~24 h later
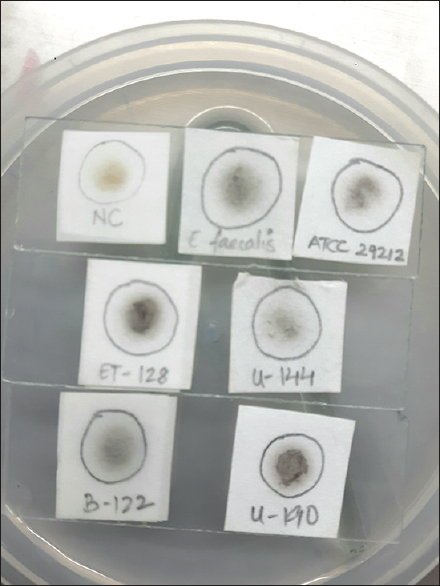
- Sodium chloride-esculin spot test. Filter paper impregnated with sodium chloride-esculin solution and placed on a standard microscope slide. Top slide: Negative control used is Staphylococcus aureus. Positive control used are Enterococcus faecalis ATCC® 29212™ and a known Enterococcus faecalis (VITEK 2 systems identified). 2nd and 3rd slide: Positive reactions are indicated by the inoculation spot turning black 30–60 minutes later
Results of Statistical analysis
In univariate analysis, risk factors for colonization or infection by enterococci were in patients with bacteremia, skin and soft-tissue infections, indwelling intravenous catheters, hospitalization (≤30 days ago), exposure to vancomycin, and multiple antibiotics [Table 4]. Patients were at greater risk of infections or colonization by VRE, HLGR enterococci, and HLSR enterococci in the presence of indwelling intravenous catheters (relative risk [RR]: 2.96, 95% CI: 1.95–4.49; RR: 2.59, 95% CI: 1.66–4.07; and RR: 2.13, 95% CI: 1.35–3.35, respectively) and in those using multiple antibiotics (RR: 2.39, 95% CI: 1.82–3.15; RR: 1.97, 95% CI: 1.43–2.72; and RR: 1.64, 95% CI: 1.18–2.30, respectively). Acquiring VRE and HLGR enterococci were associated with patients who had vancomycin exposure (VRE – RR: 3.14, 95% CI: 1.41–7.01 and HLGR enterococci – RR: 4.95, 95% CI: 2.14–11.46), and the use of aminoglycoside posed a greater risk of acquiring HLGR enterococci (RR: 1.9, 95% CI: 1.23–2.96).
| Variables | VRE isolates (n=25) | P | HLGRE isolates (n=55) | P | HLSRE isolates (n=38) | P |
|---|---|---|---|---|---|---|
| Age, mean±SD | 36.7±21.9 | 0.29 | 35.4±18.9 | 0.30 | 36.3±19.5 | 0.25 |
| Source of sample related | ||||||
| Blood samples | 8 (4.3) | <0.0001* | 11 (6.04) | <0.002* | 7 (3.8) | 0.003* |
| Urine samples | 10 (5.5) | 0.07 | 21 (11.5) | 0.0008* | 17 (9.3) | 0.05* |
| Respiratory samples | 3 (1.6) | 0.14 | 14 (7.7) | 0.94 | 8 (4.3) | 0.46 |
| Pus samples | 4 (2.2) | 0.045* | 9 (4.9) | 0.004* | 6 (3.3) | 0.03* |
| Host related | ||||||
| Diabetes mellitus | 9 (4.9) | 0.027* | 9 (4.9) | 0.39 | 10 (5.5) | 0.20 |
| Surgical procedure | 8 (4.3) | 0.27 | 23 (12.6) | 0.0002* | 13 (7.1) | 0.07 |
| Gastrointestinal disease | 3 (1.6) | 0.49 | 5 (2.7) | 0.939 | 3 (1.6) | 0.73 |
| Skin and soft tissue infection | 4 (2.2) | 0.019* | 6 (3.3) | 0.046* | 5 (2.7) | 0.03* |
| Antibiotics related | ||||||
| Multiple antibioticsa | 21 (11.5) | <0.0001* | 35 (19.2) | <0.0001* | 23 (12.6) | 0.004* |
| Vancomycin | 7 (3.8) | 0.005* | 15 (8.2) | 0.0002* | 10 (5.5) | 0.13 |
| Aminoglycosides | 5 (2.7) | 0.39 | 24 (13.2) | 0.0038* | 8 (4.3) | 0.24 |
| Cephalosporin | 9 (4.9) | 0.14 | 16 (8.8) | 0.36 | 16 (8.8) | 0.003* |
| Anaerobic drugs | 5 (2.7) | 0.24 | 23 (12.6) | 0.053 | 13 (7.1) | 0.66 |
| Hospital related | ||||||
| Recent hospitalization (≤30 days) | 13 (7.1) | <0.0001* | 14 (7.7) | 0.12 | 12 (6.6) | 0.02* |
| Recent ICU stay (≤30 days) | 4 (2.2) | 0.27 | 6 (3.3) | 0.76 | 3 (1.6) | 0.65 |
| Mechanical ventilator use | 3 (12) | 0.38 | 4 (2.2) | 0.89 | 2 (1.1) | 0.54 |
| Indwelling intravenous catheter | 3 (1.6) | <0.0001* | 27 (14.8) | <0.0001* | 18 (9.9) | 0.001* |
| Indwelling urinary catheter | 13 (7.1) | 0.12 | 22 (12.1) | 0.86 | 19 (10.4) | 0.10 |
| Outcome related | ||||||
| Length of hospital stay (days) | 7.4±5.9 | 0.018* | 9.2±4.1 | 0.0009* | 9.6±4.6 | 0.0004* |
| Prolongation of hospital stayb | 4 (2.2) | 0.02* | 7 (3.8) | 0.01* | 5 (2.7) | 0.046* |
| Death | 1 (0.5) | 0.07 | 1 (0.5) | 0.24 | 1 (0.5) | 0.67 |
Results in parentheses indicate overall percentage of infection. aMultiple antibiotic included >2 antibiotics, bProlongation of hospital stay of ≥14 days due to complications; *Significant value (P≤0.05). VRE=Vancomycin-resistant enterococci, HLGRE=High-level gentamicin-resistant enterococci, HLSRE=High-level streptomycin-resistant enterococci, SD=Standard deviation, ICU=Intensive care unit
Outcome related
Prolongation of hospitalization (due to complications during hospital stay) was seen in 16% of patients with VRE (RR: 4.19, 95% CI: 1.27–13.79), 12.7% with HLGR enterococci (RR: 5.39, 95% CI: 1.45–20.07), and 13.2% with HLSR enterococci (RR: 3.16, 95% CI: 1.02–9.79). Among them, one patient who had an isolate resistant to all the antibiotics tested died due to functional status deterioration [Table 4].
In Table 5, the logistic regression analysis revealed that the presence of an indwelling intravenous catheter was the common independent risk factor associated with resistant enterococcal infection or colonization. Antibiotic usage such as vancomycin was more likely to predispose the patients at risk to acquire HLAR enterococci than VRE, whereas multiple antibiotic uses were the risk factors associated with VRE and HLSR enterococci. History of recent hospitalization was a risk factor associated with VRE, and the presence of indwelling urinary catheter was associated with VRE and HLSR enterococci.
| Variables | VRE | HLGRE | HLSRE | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Multiple antibiotic exposure | 34.56 (9.3-128.7) | <0.0001* | 2.11 (0.8-5.5) | NS | 4.77 (1.7-13.3) | 0.003* |
| Vancomycin | 1.73 (0.5-6.5) | NS | 3.82 (1.3-11.1) | 0.013* | 5.07 (1.4-18.9) | 0.016* |
| Recent hospitalization | 10.69 (2.8-40.4) | <0.0001* | 1.42 (0.6-3.5) | NS | 2.29 (0.9-6.02) | NS |
| Indwelling intravenous catheter | 61.35 (7.9-474.1) | <0.0001* | 5.32 (2.2-13.01) | <0.0001* | 5.37 (1.9-15.2) | 0.001* |
| Indwelling urinary catheter | 12.49 (2.1-73.6) | 0.005* | 1.84 (0.8-4.1) | NS | 3.50 (1.3-9.2) | 0.011* |
aFactors associated with risk for acquiring resistant enterococci compared with sensitive enterococci, *Significant value ≤0.05. NS=Not significant, CI=Confidence interval, VRE=Vancomycin-resistant enterococci, HLGRE=High-level gentamicin-resistant enterococci, HLSRE=High-level streptomycin-resistant enterococci. OR (95% CI)=Odds ratio (95% CI)
Discussion
In this study, the prevalence of enterococcal infection was 2.7% (88/3208). Isolation of vancomycin resistance, high-level gentamycin and high-level streptomycin resistance among the Enterococcus isolated was 13.7% (25/182), 30.2% (55/182), and 20.9% (38/182), respectively. All the enterococci causing bacteremia were hospital acquired, and the presence of indwelling intravenous catheter was a common independent risk factor predisposing the patients to resistant enterococci.
In this study, a significant risk of acquiring resistant enterococci, among hospitalized patients, is attributed to exposure to multiple antibiotics, and the majority of isolates showed >30% resistance to high-level aminoglycosides in vitro. HLGR was observed more than HLSR in both groups of VRE and VSE, especially among E. faecalis which is similar to a study by Hayakawa et al.[12] Similarly, high percentages of HLAR were also reported in other studies.[131415] In this study, E. faecium was the predominant VRE isolate (48%), which is in agreement with similar studies.[161718] On the contrary, E. faecalis as the predominant VRE have been reported in other studies.[141920] Overall, isolation of HLAR was more than VRE in our study, similar to findings in other studies.[1415]
Studies on the prevalence of drug-resistant enterococci are mostly hospital based, and the implication of disease burden in the community extrapolated.[2346] The clinical significance of VRE is often difficult to ascertain (e.g., isolation from healthy individuals or when recovered in mixed cultures with other pathogens) as false-negative results confound a diagnosis.[462122] Moreover, selective identification of enterococci from only sterile body sites often overlooks the possibility of drug-resistant enterococcal colonization from other sources, thereby underestimating the burden and potential transmissibility of resistant enterococci in the facility.[623]
Although the rate of asymptomatic gastrointestinal colonization by enterococci far exceeds the rate of infection, the role of colonizers in nosocomial infections is well documented.[2456] Gut colonization, by resilient enterococci, persists for months to years, and patients often have the same organism colonizing their skin.[4] Transmission in a hospital environment can occur readily from such expanded reservoir, particularly of resistant enterococci, which explains the significant findings from our study in patients with the presence of catheters in situ, history of recent hospitalization, and increase in the duration of hospital stay by the univariate analysis.[56] Moreover, in the multivariable analysis of independent risk factors, indwelling catheters and recent hospitalization history were found to be associated with acquiring resistant enterococci. However, in this study, increase in the duration of hospital stay (>7 days) and history of recent ICU stay did not reveal any significant results (by the multivariable analysis) which are in contrast to other studies.[12192324] Studies have implicated longer duration of hospital stay to increased risk of acquiring resistant enterococci, as there are higher chances of prolongation or receiving multiple antibiotics and longer exposure time for transmission.[1924]
Various methods of enterococcal identification range from 4 to 48 hours to interpret; these are either expensive, labour-intensive or time-consuming. The NaCl-esculin hydrolysis test is a method of rapid identification with a turnaround time of 1–2 hours by the test tube method, but by the filter paper method, a positive reaction was seen within 30–60 minutes.[7] The principle behind the test is that only those organisms that hydrolyze esculin and survive the 5% NaCl environment will give a positive reaction, for example, salt tolerant, bile esculin-positive Enterococcus species.[7] Validation by comparison of the spot test with the growth of enterococci on BEA agar was because both the tests have a similar composition. However, false positive with other organisms which hydrolyze esculin should be excluded.[25] Therefore, identification of Enterococcus species must still be confirmed by other methods. Nonetheless, laboratories seeking a presumptive identification of enterococci, to distinguish it from other streptococci, could utilize this method of NaCl-esculin hydrolysis spot test. The test is relatively inexpensive and rapid and gave reliable results (among Gram-positive catalase-negative cocci).
The major limitation of this study is that only the samples of hospitalized patients were taken into consideration and hence may not be representative of organisms in the community. However, the risk of enterococcal infections/colonization is increased in a hospital due to factors, namely prevalent resilient organisms, instrumentation, indwelling catheters, and decreased host immune response, and accordingly, our study results are still meaningful and relevant.[121719]
Conclusions
To prevent nosocomial transmission of resistant enterococci, the judicious use of antibiotics, handwashing of health-care providers, isolation wards for VRE confirmed patients, and surveillance strategies have to be implemented in hospital infection control methodology.
Financial support and sponsorship
This study was funded by Dr. Ramdas Pai and Mrs. Vasanthi Pai Endowment fund SMIMS, vide letter no. SMIMS/Letter/2016/150. F. A. No: Endow/019/SMU/16-17.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to extend our gratitude to Sikkim Manipal University and the Research Unit in providing us support and financial aid to carry out this study.
References
- Routine procedures for isolation and identification of enterococci and fecal streptococci. Appl Environ Microbiol. 1992;58:3027-31.
- [Google Scholar]
- Relationships between enterococcal virulence and antimicrobial resistance. Clin Microbiol Rev. 2000;13:513-22.
- [Google Scholar]
- Enterococcus species. In: Koneman's Color Atlas and Textbook of Diagnostic Microbiology (6th ed). Philadelphia: Lippincott Williams and Wilkins; 2006. p. :700-4.
- [Google Scholar]
- Enterococcus Diversity, origins in nature, and gut colonization. 2014. Enterococci: From Commensals to Leading Causes of Drug Resistant Infection. Boston: Massachusetts Eye and Ear Infirmary; Available from: http://www.ncbi.nlm.nih.gov/books/NBK190427/
- [Google Scholar]
- Resistance mechanisms, epidemiology, and approaches to screening for vancomycin-resistant Enterococcus in the health care setting. J Clin Microbiol. 2016;54:2436-47.
- [Google Scholar]
- Sodium chloride-esculin hydrolysis test for rapid identification of enterococci. J Clin Microbiol. 1987;25:1107-8.
- [Google Scholar]
- Centers for Disease Control and Prevention. Available from: https://www.cdc.gov/hai/infectiontypes.html
- Nosocomial outbreak of vancomycin-resistant Enterococcus faecium in a paediatric unit at a Turkish university hospital. J Antimicrob Chemother. 2008;61:1033-9.
- [Google Scholar]
- Performance Standards for Antimicrobial Susceptibility Testing (26th ed). Wayne, PA: Clinical and Laboratory Standards Institute; 2016. 45, 82-5 CLSI Supplement M100S
- Identification of Enterococcus species isolated from human infections by a conventional test scheme. J Clin Microbiol. 1989;27:731-4.
- [Google Scholar]
- Epidemiology of vancomycin-resistant Enterococcus faecalis: A case-case-control study. Antimicrob Agents Chemother. 2013;57:49-55.
- [Google Scholar]
- High level aminoglycoside resistance and distribution of aminoglycoside resistant genes among clinical isolates of Enterococcus species in Chennai, India. ScientificWorldJournal. 2014;2014:329157.
- [Google Scholar]
- Emerging vancomycin resistance in enterococci in India. Indian J Pathol Microbiol. 2006;49:620-2.
- [Google Scholar]
- Prevalence of multidrug resistant enterococci in a tertiary care hospital in India: A growing threat. Open J Med Microbiol. 2014;4:11-5.
- [Google Scholar]
- Trends in susceptibility of vancomycin-resistant Enterococcus faecium to tigecycline, daptomycin, and linezolid and molecular epidemiology of the isolates: Results from the tigecycline in vitro surveillance in Taiwan (TIST) study, 2006 to 2010. Antimicrob Agents Chemother. 2012;56:3402-5.
- [Google Scholar]
- Healthcare-associated vancomycin resistant Enterococcus faecium infections in the Mansoura University hospitals intensive care units, Egypt. Braz J Microbiol. 2015;46:777-83.
- [Google Scholar]
- Characterization of vancomycin resistant enterococci in hospitalized patients and role of gut colonization. J Clin Diagn Res. 2017;11:DC01-DC05.
- [Google Scholar]
- Prevalence, outcome and risk factor associated with vancomycin-resistant Enterococcus faecalis and Enterococcus faecium at a tertiary care hospital in Northern India. Indian J Med Microbiol. 2016;34:38-45.
- [Google Scholar]
- Detection of vancomycin resistance among Enterococcus faecalis and Staphylococcus aureus. J Clin Diagn Res. 2016;10:DC04-6.
- [Google Scholar]
- Vancomycin-resistant enterococci: Colonization, infection, detection, and treatment. Mayo Clin Proc. 2006;81:529-36.
- [Google Scholar]
- High rate of false-negative results of the rectal swab culture method in detection of gastrointestinal colonization with vancomycin-resistant enterococci. Clin Infect Dis. 2002;34:167-72.
- [Google Scholar]
- 2010. Centers for Disease Control and Prevention. Available from: https://www.cdc.gov/hai/settings/lab/vreclinical-laboratory.html
- Incidence of and risk factors for infection or colonization of vancomycin-resistant enterococci in patients in the intensive care unit. PLoS One. 2012;7:e47297.
- [Google Scholar]
- Rapid spot test for the determination of esculin hydrolysis. J Clin Microbiol. 1976;4:180-4.
- [Google Scholar]





