Translate this page into:
Inflammatory pseudotumors of the liver: Importance of a multimodal approach with the insistance of needle biopsy
Address for correspondence: Dr. Chhagan Bihari, Department of Pathology, Institute of Liver and Biliary Sciences, D-1, Vasant Kunj, New Delhi - 110 070, India. E-mail: drcbsharma@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
CONTEXT:
Inflammatory pseudotumor (IPT) of the liver is a rare, tumor-like lesion that is considered to be biologically benign but often mimics malignancy.
AIMS:
The aim of the study was construe clinicopathological features, imaging findings, differential diagnosis, management, and follow-up of IPT involving the liver.
SETTINGS AND DESIGN:
It is a retrospective study.
SUBJECTS AND METHODS:
Cases included were of IPT, diagnosed on histopathology, at our center from June 2009 to December 2016. Details studied were clinical presentation, imaging studies, laboratory investigations, pathological features, treatment, and follow-up of the cases and compared with reports in the literature.
RESULTS:
A total of cases of IPT included were 17. The age of the patients ranged from 21 to 62 years. Common presenting features were intermittent fever, upper abdominal pain, and weight loss. Radiological diagnosis varied from neoplastic (13) to infectious etiologies (4), with hepatocellular carcinoma being the most common differential (7/17). Laboratory investigations revealed leukocytosis, hyperbilirubinemia, raised transaminases, and raised serum alkaline phosphatase. Core biopsy of a tumor conceded increased fibrosis along with mixed inflammatory cell infiltrates. Eleven cases were managed conservatively and showed regression or complete recovery. Six patients underwent surgical resection. None of these had any recurrence in median follow-up of 22 months.
CONCLUSIONS:
IPT of the liver can masquerade as a fatality, either primary or metastatic. It will be well managed with conservative modalities and can avoid redundant hepatectomy, reserved for complicated cases. For this intent, accurate preoperative diagnosis is the requisite, and needle biopsy with or without fine-needle aspiration cytology plays as a significant rescuer in this field.
Keywords
Antibiotics
inflammatory pseudotumor
liver
Introduction
Inflammatory pseudotumor (IPT) is a benign mass-forming lesion that can involve any organ, with the lung being the most common and liver being the second common site affected. The lack of stringent diagnostic criteria makes incidence dubious. However a retrospective analysis of 403 patients with liver surgical resection specimens, Tang L et al. found an incidence to be ~0.7% of focal liver lesions.[1] It also described as an inflammatory myofibroblastic tumor, plasma cell granuloma, histiocytoma, and fibroxanthoma.
Clinical diagnosis is difficult because of general symptoms, nonspecific imaging findings, absent serological evidence of inflammation, and presentation as space-occupying lesion (SOL). These all en masse cause diagnostic dilemma and may encompass of malignant lesions (hepatocellular carcinoma [HCC], cholangiocarcinoma, lymphoma, and metastatic tumor, etc.) and benign lesions (granulomatous, focal nodular hyperplasia [FNH], and abscess) in their differentials.
Fine-needle aspiration cytology (FNAC) too can play an essential role as an initial investigation, in the diagnosis or at least in commenting on the nature of the lesion and excluding the malignancy. Needle biopsy from the representative area will confirm the reactive nature of the injury and thus negate malignancy.[234] Invasive diagnostic and curative procedures (Laparoscopy and open hepatectomy) are often performed fortuitously under an overdiagnosis of malignancy.[56] An unnecessary hepatectomy can be avoided and reserved for complicated cases, by making an accurate preoperative diagnosis with the help of FNAC and needle biopsy. The study was done to explore various clinicopathological aspects, imaging findings, differential diagnosis, management, and follow-up of IPT involving the liver.
Subjects and Methods
Cases included were of IPT at our center, from June 2009 to December 2016. Their clinical presentations, biochemical investigations including tumor markers, and imaging studies (ultrasonography [USG], computed tomography [CT] with or without contrast, and magnetic resonance imaging [MRI]) were noted. Cytopathology, histopathology, and immunohistochemistry slides were reviewed by three pathologists (NN, SR, and CB). Treatment details and follow-up of the cases were also noted.
Results
The study comprised 17 histopathologically proven cases of IPT from a total of 12,075 hepatobiliary specimens, received in the period studied. Thirteen samples were core liver biopsies and four were of resection specimens (two cases of left hepatectomy and two of right hepatectomy). The study constituted 11 male patients and age ranged from 21 to 62 years (median: 45 years).
Clinical and biochemical examination
The patients presented with intermittent fever (11/17, 64.7%), abdominal pain (8/17, 47.0%), and weight loss (5/17, 29.4%). Laboratory investigations showed leukocytosis (8/17, 47.0%), hyperbilirubinemia (9/17, 52.9%), raised transaminases (6/17, 35.2%), raised serum alkaline phosphatase (12/17; 70.5%), and Gamma Glutamyl Transferase (GGT) (14/17; 82.3%). Autoimmune and viral markers were negative. Urine, blood culture, and amebic serology were found to be sterile. Serum tumor markers (alpha-fetoprotein [AFP], carcinoembryonic antigen [CEA], carbohydrate antigen 19-9 [CA19.9]) done showed the normal levels of serum AFP and CEA (17/17,100%); CA19.9 was done in 15 patients and came out to be increased in two patients with the values of 518.8 ng/ml and 147.4 ng/ml [relevant details summarized in Table 1].
| Cases | HB (g/dl) | WBC (103 Cells per ml) | BIL (T) (mg/dl) | AST (U/L) | ALT (U/L) | SAP (IU/L) | GGT (U/L) | AFP (<20 ng/ml) | CEA (<5 ng/ml) | CA19-9 (<37 U/ml) | Hepatitis B | Hepatitis C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 8.1 | 12.7 | 1.7 | 57 | 57 | 135 | 37 | 7 | 4.8 | 16.2 | NR | NR |
| 2 | 9.1 | 15.7 | 7.6 | 160 | 308 | 615 | 1287 | 4.6 | 8.34 | 518.8 | NR | NR |
| 3 | 12.4 | 11.9 | 0.2 | 25 | 53 | 95 | 51 | 8.2 | 5.1 | ND | NR | NR |
| 4 | 8.5 | 12.6 | 1.4 | 46 | 18 | 93 | 48 | 5.2 | 3.6 | 22.2 | NR | NR |
| 5 | 9.4 | 6.6 | 0.5 | 17 | 15 | 165 | 112 | 3.7 | 7.8 | 8.5 | NR | NR |
| 6 | 8.4 | 6.9 | 2.9 | 27 | 30 | 53 | 32 | 7.6 | 4.3 | 7.9 | NR | NR |
| 7 | 9.8 | 20.4 | 2.5 | 632 | 608 | 268 | 93 | 9.3 | 6.2 | 16.3 | NR | NR |
| 8 | 7.0 | 11.6 | 0.5 | 15 | 15 | 209 | 47 | 12.4 | 3.8 | 3.9 | NR | NR |
| 9 | 17.2 | 6.1 | 1.4 | 20 | 7 | 142 | 18 | 14.7 | 1.9 | ND | NR | NR |
| 10 | 12.7 | 19.7 | 1.1 | 44 | 40 | 212 | 84 | 4.5 | 4.1 | 16.4 | NR | NR |
| 11 | 9.3 | 7.9 | 0.7 | 25 | 33 | 109 | 57 | 3.2 | 5.42 | 1.6 | NR | NR |
| 12 | 9.4 | 8.8 | 0.6 | 26 | 17 | 49 | 12 | 11.3 | 0.89 | 39.4 | NR | NR |
| 13 | 9.5 | 8 | 0.4 | 23 | 17 | 106 | 77 | 6.8 | 1.7 | 147.4 | NR | NR |
| 14 | 10.7 | 12.8 | 3.3 | 189 | 156 | 210 | 140 | 2.9 | 3.3 | 27.1 | NR | NR |
| 15 | 12.0 | 10.8 | 1.9 | 29 | 26 | 78 | 54 | 3.1 | 9.2 | 22.8 | NR | NR |
| 16 | 11.2 | 6 | 1.5 | 23 | 15 | 105 | 115 | 3 | 0.72 | 38 | NR | NR |
| 17 | 10.9 | 6 | 0.4 | 22 | 24 | 157 | 78 | 5.3 | 2.85 | 11.8 | NR | NR |
AFP=Alpha feto protein, CEA=Carcinoembryonic antigen, CA19-9=Carbohydrate antigen 19-9, SAP=Serum alkaline phosphatase, WBC=White blood cell, NR=Nonreactive, HB=Haemoglobin, BIL=Bilirubin, AST= Aspartate Transaminase, ALT=Alanine Transaminase, GGT =Gamma Glutamyl Transferase
Radiological investigations
Imaging studies revealed the preponderance of cases having hepatomegaly (12/17; 70%), solitary (14/17; 82.4%), and space-occupying lesion (13/17; 76.5%) with well-defined (10/13) borders and right lobe involvement (11/17; 64.7%). Four cases (4/17; 23.5%) showed an infiltrative pattern. On USG, the lesions were hypoechoic (13) to isoechoic (4). Contrast-enhanced computer tomography has done (15) which showed hypodense lesion with avid arterial enhancement with washout in venous and delayed phase (4) and heterogeneously enhanced lesions with peripheral rim enhancement (7) and central hypoattenuation (5). MRI done (6) showed hypointense lesion on T1 and hyperintense signal intensity on T2-weighted images with diffusion restriction. Additional findings include multiple intra-abdominal lymph nodes (5), splenomegaly (4), and portal vein thrombosis (2) with cavernoma formation (2) [Figures 1 and 2].
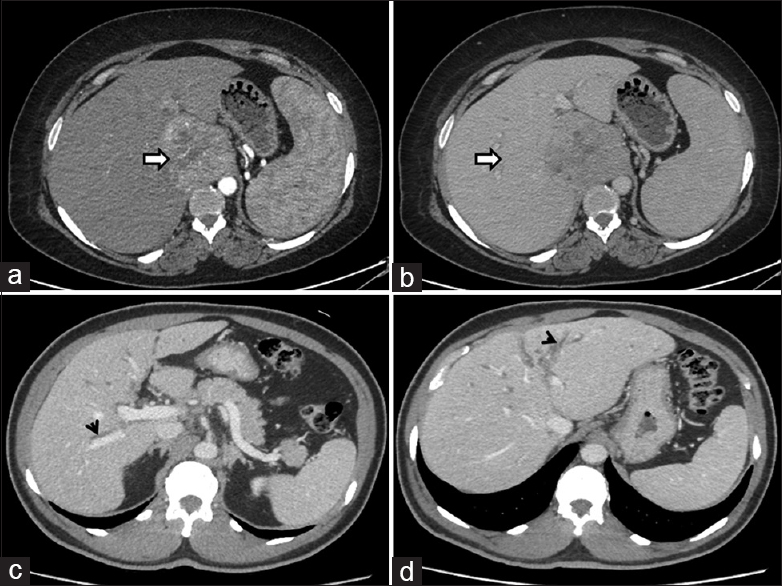
- Axial contrastenhanced computed tomography images of one case with (a) arterial phase showing hypervascular lesion (arrow) in the caudate lobe and (b) washout in venous phase (arrow) compressing the IVC mimicking HCC; Axial contrastenhanced computed tomography images of another case (c,d) showing periductal thickening involving the bilateral intrahepatic bile ducts (arrowheads) mimicking CCA

- Contrastenhanced magnetic resonance imaging of the abdomen showing (a) T2 hyperintense lesion (arrow) in segment VI of the liver (b) heterogeneous post-contrast enhancement and delayed rim enhancement (arrow), (c,d) is the diffusionweighted and ADC images, showing diffusion restriction with low ADC values and mimicking Hepatocellular Carcinoma; Coronal contrastenhanced computed tomography image (e) of a complicated case showing a welldefined exophytic heterogeneously enhancing mass lesion (arrow) in segment III of the liver having non-enhancing cystic/necrotic component abutting pyloric antrum of the stomach. Incidental note made of pneumobilia (arrowhead)
Two cases display an unusual presentation: one with extension into the gallbladder fossa, infiltrating into the omentum and adjacent tissues with an intraoperative observation of perihepatic adhesions and other with an exophytic component, abutting lesser curvature of the stomach, hence raising suspicion of periductal hilar cholangiocarcinoma [Figure 2e].
The radiological diagnoses varied from neoplastic (13) to infectious etiologies (4), with differentials being HCC (7, 41.2%), metastasis (2,11.8%), cholangiocarcinoma (2,11.8%), lymphoma (1, 5.9%), FNH (1, 5.9%), granulomas (2,11.8%), liver abscess (1, 5.9%), and portal cavernoma (1, 5.9%).
Cytomorphological examination
FNAC available in 8 cases showed mixed inflammatory infiltrate (8), plasma cell prominence (6), aggregates of neutrophils (5), foamy histiocytes (5), ill-defined epithelioid cell collection (3), multinucleated giant cells (2), necrosis (3) and sheets of benign hepatocytes. Ziehl–Neelsen stain for acid-fast bacilli and periodic acid–Schiff stain for fungal profiles were found negative in all cases. An isolated case showed atypical cells with enlarged pleomorphic nuclei with coarse chromatin, conspicuous nucleoli, and scant cytoplasm, raising the possibility of malignancy and subsequently leading to the surgical excision [Figure 3].

- Fine-needle aspiration cytology showed occasional granuloma formation (a) (arrow), mixed inflammatory infiltrate (b), and occasional mild atypia (c and d) (arrow), (H and E, Giemsa × 400)
Histopathological examination
Microscopic examination of liver mass (core biopsy: 13 and surgical resection specimens: 4) showed well- to ill-defined lesions with similar histomorphology. Spindle cells to stellate cells were arranged in short fascicles and whorls in a dense collagenous background (17), interspersed intense inflammatory infiltrate [Figure 4a-d] with prominent plasma cell infiltrate >20 plasma cells/HPF, and increased eosinophilic infiltration >5/hpf was noted. There were clusters of xanthomatous cells (12) with epithelioid cell granulomas (6), multinucleated giant cells (5), and areas of necrosis (7) [Figure 5a-d]. Furthermore, obliterative phlebitis was observed in two cases.

- Histopathology sections showed large sheets of plasma cells (a) (arrow), mixed inflammation (b), increased eosinophils (c) (arrow), and neutrophils (d) (H and E, 400)

- Histopathology revealed granulomas (a and b) (H and E, 400). Immunohistochemistry showed IgG4 positive plasma cells (c) (arrow) and smooth muscle actin is positive in myofibroblastic cells
Immunohistochemical examination
On immunohistochemistry, spindle cells were positive for vimentin and smooth muscle actin and negative for desmin, CD34, and S100. CD68 was positive in histiocytes. IgG4 was done in 15 cases and found positive in two cases (IgG4 >10/hpf) and diagnosed as IgG4-related IPT given additional findings of obliterative phlebitis and storiform fibrosis [Figure 6].

- There are increased foamy macrophages (a) along with a few giant cells (b) (arrow). Myofibroblastic proliferation (c) and obliterative phlebitis (d, arrow) is also noted (H and E; ×400)
Treatment and follow-up
Four were resection specimens, and surgical interventions were performed in additional two cases with partial hepatectomy in one and an extended hepatectomy with common bile duct (CBD) resection and lymph node clearance in another case. The remaining patients (11; 64.7%) were treated conservatively with antibiotics and/or anti-inflammatory drugs.
Cases were followed up for a median duration of 22 months. Twelve patients showed complete recovery of the lesion with no episode of recurrence. Ten of eleven conservatively managed patients found to fare well with complete recovery or regression of lesion on follow-up. After 23 months of follow-up, a single patient expired which on conservative treatment modalities. Five cases among that underwent resection (6) showed no recurrence. One patient has died postoperatively [case-wise details summarized in Table 2].
| Case | Age (years) | Sex | Presentation | Site | Size (cm) | Circumscription | Pattern | Solitary/multiple | Radiological diagnosis | Treatment | Follow up (months) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 43 | Female | Fever, pain abdomen | Right | 6.0×6.0 | Ill defined | SOL | Solitary | Portal cavernoma | Antibiotics | 25 | Recovery |
| 2 | 55 | Male | Fever, weakness, malaise, jaundice | Right | 7×2.5 | Ill defined | Infiltrative | Solitary | Cholangiocarcinoma | Resection | 2 | Expire |
| 3 | 42 | Male | Fever, pain abdomen | Right | 5.3×5.3 | Well defined | SOL | Solitary | HCC | Resection | 38 | Recovery |
| 4 | 42 | Male | Fever, fatigue, dizziness | Right | 3.5×3 | Well defined | SOL | Solitary | FNH | Antibiotics | 23 | Expire |
| 5 | 42 | Female | Pain abdomen, fever | Left | 9.2×7.7 | Well defined | SOL | Solitary | HCC | Resection | 6 | Regression |
| 6 | 46 | Male | Jaundice, dizziness, weight loss | Right | 7×8 | Well defined | SOL | Multifocal | HCC | Antibiotics | 40 | Recovery |
| 7 | 49 | Male | Fever, nausea, jaundice, weight loss | Right | 11.5×10 | Well defined | SOL | Solitary | HCC | Resection | 38 | Recovery |
| 8 | 33 | Male | Fever, indigestion | Right | 6.3×4.6 | Infiltrative | Infiltrative | Solitary | Abscess | Antibiotics | 37 | Recovery |
| 9 | 21 | F | Pain abdomen, nausea, vomiting | Right | 1.4×2.4 | Ill defined | SOL | Solitary | Granulomatous | Antibiotics, antiinflammatory | 27 | Recovery |
| 10 | 43 | Male | Fever, pain abdomen | Left | 4.6×3.9 | Well defined | SOL | Solitary | Metastasis | Antibiotic | 26 | Recovery |
| 11 | 60 | Female | Pain abdomen, weight loss | Left | 8.7×6.3 | Infiltrative | Infiltrative | Solitary | HCC | Resection | 29 | Recovery |
| 12 | 40 | Male | Indigestion, nausea, vomitting | Right | 3.2×2.6 | Well defined | SOL | Solitary | HCC | Antibiotic | 4 | Recovery |
| 13 | 60 | Female | Vomitting, weight loss | Right | 2.6×2.4 | Well defined | SOL | Multifocal | Granulomatous | Antibiotics, antiinflammatory | 13 | Recovery |
| 14 | 58 | Male | Fever, jaundice | Left | 8.0×6.1 | Ill defined | Infiltrative | Solitary | Cholangiocarcinoma | Antibiotic | 7 | Regression |
| 15 | 45 | Female | Fever, nausea | Right | 5.0×4.7 | Ill defined | SOL | Solitary | Lymphoma | Antibiotic | 6 | Regression |
| 16 | 62 | Male | Pain abdomen, fever | Left | 4.4×3.4 | Ill defined | SOL | Multifocal | Metastasis | Resection | 10 | Recovery |
| 17 | 62 | Male | Pain abdomen, fatigue, weight loss | Left | 6.0×4.8 | Exophytic | SOL | Solitary | HCC | Antibiotic | 18 | Recovery |
SOL=Space occupying lesion, FNH=Focal nodular hyperplasia, HCC=Hepatocellular carcinoma
Discussion
“IPT” is a diverse group of mass-forming lesions that can grip any organ and is peculiarized by an inflammatory infiltrate as the predominant cellular component.
Eastern countries show the older age of onset in contrast to studies from Western countries which have reported a higher incidence in mid-thirties and the male-to-female ratio of 1–3.5:1.[78] The median age of patients in this study was ~45 years with a male: female ratio of 1.8:1, which is a bit earlier presentation as found by Park et al. in a 3-year study with 15 patients having mean age of 60.3 ± 9.2 years and Ahn et al. in a study comprising 22 patients of IPT liver exhibiting median age IPT liver found to be 59 years. The male-to-female ratio was 2:1 and 2.6:1, respectively.[910]
Intermittent fever, pain abdomen, and loss of weight were the prevailing symptoms in the study. A similar set of symptoms found by Tang et al. in a retrospective search of 64 patients underwent partial hepatectomy in suspicion of malignancy. The other presentations may include obstructive jaundice, splenomegaly, and portal hypertension.
Etiology and pathogenesis remain unknown for this lesion. A review article enlightened the pathogenesis of IPT as an exaggerated inflammatory process and concluded the possible etiologies being infectious agents, autoimmune reactions, and systemic inflammatory response syndrome.[11] Hence, laboratory investigations often indicate an ongoing inflammatory process with increased inflammatory markers including erythrocyte sedimentation rate, C-reactive protein, leukocytosis, and deranged liver function tests, as noted in this study.[12]
Tumor markers including CEA and AFP are usually regular in IPT, whereas mild raise in CA19-9 could be noted.[1314] Increased CA19-9 and a hilar mass lesion in two patients harbor the clinical suspicion of cholangiocarcinoma. A case report of a 50-year-old Japanese male diagnosed with IPT liver associated with raised CA19-9 elucidated the biliary epithelium involvement by inflammatory cells and narrowed portal canals, leading raise of CA19-9.[15]
Solitary SOL being the standard presentation, consistent with the study, and only scares data in the literature described the multicentricity in the form of few case reports, as described by Weiss et al. in a 79-year-old male.[16]
IPT has conceded hypoechoic, as well as hyperechoic, masses on USG. The CT scan usually reveals lesions with variable contrast enhancement. IPTs with increased fibrosis show hypovascularity with delayed enhancement, similar to metastatic liver tumors and cholangiocarcinomas.[14] On MRI, the lesions are hypointense on T1-weighted images and hyperintense on T2-weighted images, with subtle enhancement patterns. Fukuya et al. reviewed CT findings of 9 diagnosed cases of IPT liver, and Park et al. did a study with multicentre exposure of 45 cases of IPT. They both suggest to include IPT as differential when liver mass is reasonably large, solitary, and contrast enhancement is more significant than liver parenchyma on delayed phase CT scan. They recommended percutaneous needle biopsy for the confirmation of diagnosis.[1718]
Indifferent imaging findings may lead to an expansive range of pathologies in their differentials, varies from malignant lesions (lymphoma, malignant fibrous histiocytoma, HCC, metastatic tumor, and cholangiocarcinoma, etc.) to benign lesions such as an abscess, FNH, and granulomatous lesions (tuberculosis and sarcoidosis).
Very scarce literature with few case reports describing fine-needle aspiration (FNA) findings in these lesions make this case series one of the very few, describing these findings in diagnosed cases of IPT on subsequent histopathological evaluation. Hosler et al. describe a case series of 12 cases from 8 patients with histologically proven cases of IPT. They found mixed inflammatory infiltrated with fibroblastic proliferation and increased mitosis which may mimic mesenchymal neoplasm. The authors found that cytomorphology is nonspecific and can be useful in excluding the possibility of malignancy.[19] As all the cases, in which FNAC was done, showed similar findings, it seems to play an essential role in the diagnosis or at least in commenting on the nature of the lesion and hence on the line of treatment. One of our cases shows atypical epithelial cells on cytology and hence leads to surgical resection of the lesion. The case reported by Lupovitch et al. defined the limited role of FNAC in the diagnosis of IPT as it causes a diagnostic dilemma. They stated that initial FNA findings were those of an acute exudative process with atypical biliary duct epithelium or hepatocytes and hence mislead the diagnosis.[20]
Recently, IPT has been classified pathologically into two types: fibrohistiocytic and lymphoplasmacytic.[21] Fibrohistiocytic subtype comprises xanthogranulomatous inflammation, multinucleated giant cells, and neutrophilic infiltration. They occur predominantly in the peripheral hepatic parenchyma as a mass-forming lesion. In contrast, the lymphoplasmacytic subtype is found exclusively around the hepatic hilum and is composed of diffuse lymphoplasmacytic infiltration and prominent eosinophilic infiltration. A lymphoplasmacytic subtype is commonly associated with obliterative phlebitis and cholangitis with periductal fibrosis and is less common in fibrohistiocytic type. A significant amount of IgG4-positive plasma cells (>10/hpf in core biopsy or surgically resected specimen) are seen in the lymphoplasmacytic type. Immunohistochemically, the bulk of the cases involved (15/17) showed IgG4-negative plasma cells and classified into the fibrohistiocytic type.[21]
Prognosis of the lesion has been considered to be good with conservative and surgical treatment modalities. Rare instances of multiple IPTs, aggressive behavior, with multiple recurrences, invasion into adjacent structures, and metastases have been reported in the literature as shown by Coffin et al. and Walsh et al.[2223] We too had a case that expired in their near future due to aggressive and infiltrative behavior and large size of the mass. A 55-year-old male who was a known case of diabetes mellitus and hypertension presented with complaints of fever and yellowish discoloration of urine. The radiological and clinical diagnosis was hilar cholangiocarcinoma for which hepatectomy was performed. Perihepatic adhesions were observed intraoperatively. He had two episodes of right portal vein embolism and required surgical intervention.
Another patient was a 42-year-old male with a history of uncontrolled diabetes mellitus from the past 6 years and hypertension. He was an alcoholic and had chronic liver disease (ethanol related), portal hypertension, decompensated with ascites, and Grade III hepatic encephalopathy. He also had chronic kidney disease and episodes of nonconvulsive seizures. After 23 months of follow-up, the lesion was regressed, but the patient died because of other comorbidities.
Calomeni et al. described a case series of four patients with intrahepatic mass and were treated conservatively by antibiotics found no response and eventually lead to surgical resection. They found conservative management as the first-line treatment, although surgery is often necessary.[24] Contrast to the study mentioned above, Goldsmith et al. analyze 10 cases, and a comprehensive review of the published literature including 215 cases of IPT liver emphasizes the medical treatment with a good prognosis on follow-up. The recommendation was surgical resection because of unclear pathological diagnosis or when is not responding to conservative management.[25] All of the patients with conservative or surgical treatment show regression/complete resolution and recurrence was not found, after the median follow-up of 22 months. Two patients expired despite proper management protocols.
IPT is accepted as a benign entity with the plausibility of infiltration and recurrence. Literature suggests that percutaneous tumor biopsy may provide the correct diagnosis and on treatment with antibiotics and/or corticosteroids will lead to complete resolution of the lesion.[26]
Needle biopsy with or without FNA will do a great help in the definitive diagnosis of IPT. In such cases, antibiotic/or corticosteroids treatment may be curative, and hepatic resection can be evaded. In a few instances, it can also regress spontaneously.
In the absence of a firm diagnosis of a resectable hepatic mass, excision is a well-known practice followed by the surgeons. All the same, if a preoperative diagnosis of an IPT can be made, we can revolute the conventional practice of surgical resection towards the conservative management approach.
What is already known?
-
IPTs are common mimics of hepatic malignancy
-
Often treated by surgical hepatectomy
-
This group comprises multifarious lesions with common histology of inflammatory infiltrate.
What are the new findings?
-
Comprehensive findings to diagnose these lesions on cytopathology
-
These can be managed conservatively if the preoperative diagnosis is established on cytology or needle biopsy.
Conclusions
These nonspecific clinical symptoms and variable radiological appearances on imaging pose a great challenge and stress the need for accurate diagnosis of the tumor to avert superfluous hepatectomy and reserved for complicated cases. This study delineates the prerequisite of considering IPT if an atypical mass is found in the liver, particularly when a clinical inflammatory process accompanies it. A needle biopsy with or without FNAC should be routinely practiced in undiagnosed hepatic masses.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Inflammatory myofibroblastic tumor of the liver: A cohort study. World J Surg. 2010;34:309-13.
- [Google Scholar]
- Aggressive manifestations of inflammatory pulmonary pseudotumor in children. Pediatr Radiol. 1999;29:112-6.
- [Google Scholar]
- Inflammatory pseudotumor of the liver masquerading as hepatocellular carcinoma after a hepatitis B virus infection: Report of a case. Surg Today. 2006;36:1028-31.
- [Google Scholar]
- Inflammatory pseudotumor of the liver in a patient with chronic hepatitis C: Difficulty in differentiating it from hepatocellular carcinoma. Pathol Int. 1999;49:726-30.
- [Google Scholar]
- Inflammatory pseudotumor of the liver associated with extrahepatic infection. South Med J. 1997;90:23-9.
- [Google Scholar]
- Inflammatory pseudotumor in the liver associated with intrahepatic bile duct stones mimicking malignancy. J Nippon Med Sch. 2009;76:154-9.
- [Google Scholar]
- Inflammatory pseudotumor of liver – A clinical review of 15 cases. Korean J Hepatol. 2006;12:429-38.
- [Google Scholar]
- Inflammatory pseudotumors mimicking intrahepatic cholangiocarcinoma of the liver; igG4-positivity and its clinical significance. J Hepatobiliary Pancreat Sci. 2012;19:405-12.
- [Google Scholar]
- Inflammatory pseudo-tumor of the liver: A rare pathological entity. World J Surg Oncol. 2011;9:5.
- [Google Scholar]
- Inflammatory pseudotumor of the liver. Report of four cases and review of the literature. Am J Surg Pathol. 1993;17:231-8.
- [Google Scholar]
- Intrahepatic cholangiocarcinoma mimicking hepatic inflammatory pseudotumor. J Gastrointest Surg. 2007;11:398-402.
- [Google Scholar]
- Inflammatory pseudotumor of the liver: CT and sonographic findings. AJR Am J Roentgenol. 1996;167:485-7.
- [Google Scholar]
- A case of inflammatory pseudotumor of the liver causing elevated serum CA19-9 levels. Am J Gastroenterol. 1998;93:2551-5.
- [Google Scholar]
- Inflammatory pseudotumor of the liver: An unlikely cause of multiple hepatic lesions. Isr Med Assoc J. 2007;9:894-5.
- [Google Scholar]
- Diagnosis of inflammatory pseudotumor of the liver: Value of CT. AJR Am J Roentgenol. 1994;163:1087-91.
- [Google Scholar]
- Clinical features, image findings, and prognosis of inflammatory pseudotumor of the liver: A multicenter experience of 45 cases. Gut Liver. 2014;8:58-63.
- [Google Scholar]
- Inflammatory pseudotumor: A diagnostic dilemma in cytopathology. Diagn Cytopathol. 2004;31:267-70.
- [Google Scholar]
- Inflammatory pseudotumor of the liver. Report of the fine needle aspiration cytologic findings in a case initially misdiagnosed as malignant. Acta Cytol. 1989;33:259-62.
- [Google Scholar]
- Pathological classification of hepatic inflammatory pseudotumor with respect to IgG4-related disease. Mod Pathol. 2007;20:884-94.
- [Google Scholar]
- Extrapulmonary inflammatory myofibroblastic tumor: A clinical and pathological survey. Semin Diagn Pathol. 1998;15:85-101.
- [Google Scholar]
- Inflammatory myofibroblastic tumor of the pancreaticobiliary region: Morphologic and immunocytochemical study of three cases. Am J Surg Pathol. 1998;22:412-8.
- [Google Scholar]
- Hepatic inflammatory pseudotumor: A case series. Int J Surg Case Rep. 2013;4:308-11.
- [Google Scholar]
- Inflammatory pseudotumours of the liver: A spectrum of presentation and management options. Eur J Surg Oncol. 2009;35:1295-8.
- [Google Scholar]
- Regression of inflammatory pseudotumor of the liver under conservative therapy. Dig Dis Sci. 1995;40:752-6.
- [Google Scholar]





