Translate this page into:
Clinical implication of changes in serum cations and anions on clinical severity in sickle cell disease: A case–control study in a tertiary center
*Corresponding author: Suprava Patel, MD, Department of Biochemistry, All India Institute of Medical Sciences, Raipur, Chhattisgarh, India dr_suprava@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: Patel S, Chandrakar D, Wasnik PN, Nanda R, Mohapatra E. Clinical implication of changes in serum cations and anions on clinical severity in sickle cell disease: A case–control study in a tertiary center. J Lab Physicians. 2024;16:89-96. doi: 10.1055/s-0043-1772684
Abstract
Objectives:
Vaso-occlusive crisis in sickle cell disease (SCD) is related to disproportionate serum ions distribution. The objective was to evaluate the influence of changes in serum cations and anions on clinical severity in SCD patients.
Materials and Methods:
The case–control study included 26 SCD cases and 27 apparently healthy control individuals. The cases were further grouped as crisis state and steady state cases. Each participant was assigned a disease severity score. All study participants were evaluated for serum electrolytes, osmolality, calcium, magnesium, phosphorus, total protein, and albumin.
Statistical Analysis:
IBMSPSS version 26 was used for the statistical analysis.
Results:
The mean serum sodium (135.65 mmol/L), chloride (99.88 mmol/L), calcium (2.28 mmol/L), magnesium (0.76 mmol/L), osmolality (285.19 mOsm/kg), and albumin (0.59 mmol/L) were grossly reduced in cases than the control group. The SCD crisis group recorded low serum sodium (p = 0.01), calcium (p = 0.011), total cations (p = 0.023), anions (p = 0.008), and cation–anion ratio (p = 0.041). Of all the cations, serum calcium correlated inversely with disease severity score (r = –0.367, p = 0.033), whereas serum albumin among the serum anions influenced severity the most (r = –0.338, p = 0.046). The cutoff values for serum calcium and albumin were, respectively, 2.41 and 0.63 mmol/L, with a sensitivity of 83.3 and 88.9%. A calculated ratio of serum sodium–albumin revealed a positive relationship with the severity score (r = 0.328, p = 0.05) with a sensitivity of 94.4% for a cutoff value of 208.45.
Conclusion:
Low serum sodium, calcium, albumin, and raised sodium–albumin ratio were linked to the crisis state, and routine estimation of these parameters will help in the early assessment of the clinical severity and initiate appropriate fluid management.
Keywords
Serum electrolytes
Osmolality
Crisis state
Steady state
Disease severity score
Calcium
Magnesium
Albumin
INTRODUCTION
Sickle cell disease (SCD), an autosomal recessive hemoglobinopathy, is characterized by sickle-shaped red blood cells (RBCs). The most common complications in these patients are chronic hemolysis, microvascular occlusion, and tissue damage, and all stem from the tendency of sickle cell hemoglobin (HbS) to polymerize when deoxygenated.[1,2] Repeated bouts of hypoxia and dehydration make red cells stiff and sticky. An increase in stiffness leads to splenic entrapment and extravascular hemolysis leading to microvascular occlusion. Such repeated events cause ischemia-reperfusion insult to organs leading to organ damage.[1,3] Recent articles reported that such abnormality could impair the potassium–chloride (K+-Cl−) cotransport mechanism and the calcium (Ca2+)activated Gardos channel in RBC membranes responsible for the intraerythrocytic shift of K+.[4] In deoxygenated state, the RBC membranes become permeable to Ca2+ and permeable cation channels are activated, and the Ca2+ current flows into the cytosol. Rapid Ca2+ influx of ions into the cell causes abnormal ion channel activation, leading to the efflux of K+ ions and water from the RBCs.[4-7] Thus, sickling raises intracellular Ca2+ and loss of K+ and water from red cells, thereby increasing K+ levels in the blood. K+ is the principal cation for heart and muscle function; its rise will lead to abnormal heart function and muscle weakness. An altered sodium–potassium ATPase (Na+-K+ ATPase) function, often seen in sickle cell patients, also creates serum electrolyte imbalance.[3] A decrease in serum sodium (Na+) will impair nerve impulse conduction.[3] Na+, one of the major cations of the extracellular space, is the crucial regulator of water homeostasis. Besides, it is critical in the muscular system and nerve conduction. Along with Na+, chloride (Cl−) also helps maintain body fluid. Alterations of these fluid ions might result in altered consequences.[3] Intracellular dehydration in these patients increases mean corpuscular hemoglobin concentration (MCHC), which increases the probability of aggregation and polymerization during any given period of deoxygenation. SCD patients suffer from frequent episodes of hemolysis, managed mainly by repeated blood transfusions leading to electrolyte imbalance, most often hyperkalemia. Changes in plasma osmolality, either due to fluid overload during intravenous therapy or kidney dysfunction, leads to RBC dehydration, intracellular HbS concentration, and polymerization in the deoxygenated state, further promoting sickling.[1,3,8] The extracellular tonicity alters the RBCs’ biomechanical properties; thus, managing these patients with suitable intravenous fluids of appropriate volume and flow rate would most likely improve the rheological properties and reduce the frequency of crisis events.[9] Presently, Hb and fetal Hb (HbF) is routinely measured to assess the clinical course.[10] HbF is routinely measured in highperformance liquid chromatography (HPLC) technique which is not only costly but also not feasible in all health care centers so that patients could be monitored. Therefore, monitoring the clinical course using markers that could be easily measured at all health care facilities would be more convincing. The study aimed to understand the ongoing changes in serum cations and anions and their impact on clinical severity in SCD patients. It is hypothesized that regular monitoring of serum ions will reduce mortality and morbidity and increase sickle cell patients’ life expectancy.
MATERIALS AND METHODS
The case–control study was conducted on 53 adult participants attending the sickle cell clinic of the institute. The study participants comprised 26 cases and 27 apparently healthy control individuals. The cases included all homozygous SCD (HbSS) diagnosed individuals after HPLC confirmation in the Variant-II Hemoglobin Testing System (BioRad). The subjects were further grouped as crisis state (n = 18) and steady state cases (n = 8) to understand the differences in serum parameters. Individuals presenting with either of the symptoms of crisis, as depicted in Table 1, were grouped under crisis state, and those who attended the sickle cell clinic for follow-up and had no such symptoms were grouped under steady state. Cases with a history of blood transfusion in the last 2 weeks were excluded to avoid the confounding results of biochemical changes associated with transfusion. The clinical details were recorded as per the validated questionnaire used in the clinic. As per the presenting symptoms, each participant grouped under cases was assigned a score as per the severity scoring system from Adegoke and Kuti.[11,12] The scores were added, and a disease severity score was calculated for each. Blood samples were collected per the aseptic protocol in a plain vacutainer with a clot activator (red cap). Serum was analyzed for electrolytes (sodium, potassium, and chloride) by ion-selective electrode modules, calcium by Arsenazo III method, phosphorus by modified Daly and Ertingshausen method, magnesium by xylidyl blue method, total protein by modified Weichselbaum method, and albumin by bromocresol green method processed in AU 5811 fully automated autoanalyzer from Beckman Coulter. Serum osmolality was measured using the principle of freezing point depression in Advanced Osmometer (3250) (Advanced Instruments, Massachusetts, United States). Calculations for ratios and conversion to mmol/L were performed as given in Table 2.[13-15]
| Crisis events | Clinical symptoms |
|---|---|
| Acute pain | Acute onset of severe pain in the chest, abdomen, back, long bones, hands and feet, dactylitis |
| Severe chronic pain | Persistent pain due to leg ulcers, avascular necrosis (femoral or humeral head) |
| Acute chest syndrome | Pleuritic chest pain or pain in ribs, sternum and hemoptysis or features of acute respiratory illness likefever, productive cough, wheezing, dyspnea |
| Features of stroke | Progressive neurocognitive deficits like poor academic performances or any acute neurological deficits,sudden occurrence of severe headache, vomiting, seizures, speech and altered sensorium |
| Acute splenic sequestration crisis | Sudden and rapid enlargement of spleen associated with reduction of hemoglobin (Hb) |
| Features of hypovolemic shock | Worsening anemia associated with tachycardia, hypotension, and lethargy |
| Features of infection and sepsis | High grade fever, positive culture in body fluids, raised biomarkers suggestive for infection |
| Features of organ failure | Acute or chronic renal failure or multisystem organ failure like nonfocal encephalopathy/retinopathy/pyrexia of unknown origin (PUO) |
| Total cations: sum of all measured serum cations such as sodium, potassium, calcium, magnesium in mmol/L |
| Total anions: sum of all measured serum anions such as chloride, phosphorus, albumin in mmol/L |
| Conversion factor for serum calcium in mg/dL to mmol/L: calcium (mg/dL)m 0.25 mmol/L |
| Conversion factor for serum phosphorus in mg/dL to mmol/L: phosphorus (mg/dL)m 0.3229 mmol/L |
| Conversion factor for serum magnesium in mg/dL to mmol/L: magnesium (mg/dL)m 0.4114 mmol/L |
| Conversion factor for serum albumin in g/dL to mmol/L: albumin (g/dL)m 0.1505 mmol/L |
The mutual relationships of the serum variables with each other and the impact on clinical severity, various ratios were considered during analysis like sodium–potassium ratio, sodium–chloride ratio, calcium–phosphorus ratio (CPR), calcium–magnesium ratio (CMR), cation–anion ratio (CAR), and sodium–albumin ratio (SAR). Since serum osmolality is a net effect of all cations and anions, including albumin in serum, ratios of osmolality with these serum variables were calculated, such as osmolality–sodium ratio (OSR), osmolality– albumin ratio (OABR), osmolality–cation ratio, and osmolality–anion ratio (OAR).
Statistical analysis
IBMSPSS version 26 was used for the statistical analysis. The quantitative variables were checked for normality, and the study groups’ values were compared by independent samples’ t-test [Tables 3 and 4]. The influence of the serum variables on disease severity score in cases, as depicted in Table 5, was analyzed by linear regression analysis. The serum variables in cases were analyzed by the receiver operating characteristics (ROC) curve and area under the curve (AUC) for the occurrence of the crisis event. From the ROC analysis, the cutoff values with the highest sensitivity for a case to present with a crisis state were determined and tabulated in Table 6 Figures 2 and 3. The frequency percentages of cases with normal and abnormal serum values were calculated and delineated in Figure 1. The distribution pattern between the crisis and steady states of the cases were compared using chi-square test and the odds ratio (OR) with a 95% confidence interval (95% CI).
| Parameters (units) | Cases N = 26 | Control N = 27 | p-Value |
|---|---|---|---|
| Mean (SD) | |||
| Age (y) | 22.19 (6.23) | 22.85 (2.58) | 0.25 |
| Sodium | 135.65 (4.17) | 140.56 (2.03) | 0.005a |
| Potassium | 4.31 (0.6) | 4.24 (0.42) | 0.101 |
| Chloride | 99.88 (5.9) | 103.44 (2.06) | 0.001a |
| Sodium–potassium ratio (SPR) | 32.02 (4.3) | 33.45 (3.2) | 0.17 |
| Sodium–chloride ratio (SCR) | 1.36 (0.05) | 1.36 (0.02) | 0.013a |
| Osmolality | 285.19 (9.15) | 292.04 (6.09) | 0.068 |
| Calcium | 2.28 (0.2) | 2.45 (0.13) | 0.027a |
| Phosphorus | 1.39 (0.32) | 1.2 (0.2) | 0.08 |
| Magnesium | 0.76 (0.12) | 0.86 (0.07) | 0.004a |
| Calcium–phosphorus ratio (CPR) | 1.72 (0.47) | 2.12 (0.5) | 0.55 |
| Calcium–magnesium ratio (CMR) | 3.07 (0.5) | 2.85 (0.28) | 0.007a |
| Total protein | 7.45 (1.01) | 7.89 (0.47) | < 0.001a |
| Albumin | 0.59 (0.11) | 0.69 (0.05) | 0.001a |
| Cation–anion ratio (CAR) | 1.44 (0.06) | 1.43 (0.02) | 0.013a |
| Cations | 143.01 (4.2) | 148.1 (2.1) | 0.011a |
| Anions | 101.87 (5.9) | 105.34 (2.02) | 0.001a |
| Sodium–albumin ratio (SAR) | 239.59 (46.6) | 204.3 (12.6) | 0.001a |
| Osmolality–sodium ratio (OSR) | 2.1 (0.06) | 2.08 (0.05) | 0.21 |
| Osmolality–albumin ratio (OABR) | 504.72 (104.1) | 424.51 (27.3) | < 0.001a |
| Osmolality–cations ratio (OCR) | 1.99 (0.06) | 1.97 (0.04) | 0.115 |
| Osmolality–anions ratio (OAR) | 2.85 (0.15) | 2.81 (0.07) | 0.005a |
Abbreviation: SD, standard deviation.
aSignificant at p value<0.05.
| Parameters | Crisis N = 18 | Steady state N = 8 | p-Value |
|---|---|---|---|
| Mean (SD) | |||
| Age (y) | 23.33 (7.2) | 19.63 (0.7) | 0.012a |
| Sodium | 135 (4.8) | 137.13 (1.6) | 0.01a |
| Potassium | 4.19 (0.6) | 4.58 (0.7) | 0.302 |
| Chloride | 99.8 (7.1) | 100.13 (1.8) | 0.008a |
| Sodium–potassium ratio (SPR) | 32.7 (4.2) | 30.48 (4.4) | 0.72 |
| Sodium–chloride ratio (SCR) | 1.36 (0.06) | 1.37 (0.02) | 0.07 |
| Osmolality | 285.50 (10.7) | 284.5 (4.5) | 0.067 |
| Calcium | 2.21 (0.19) | 2.45 (0.06) | 0.011a |
| Phosphorus | 1.46 (0.36) | 1.27 (0.13) | 0.002a |
| Magnesium | 0.72 (0.12) | 0.86 (0.07) | 0.104 |
| Calcium–phosphorus ratio (CPR) | 1.63 (0.5) | 1.94 (0.2) | 0.049a |
| Calcium–magnesium ratio (CMR) | 3.16 (0.5) | 2.87 (0.3) | 0.036a |
| Total protein | 7.13 (0.99) | 8.18 (0.58) | 0.13 |
| Albumin | 0.535 (0.08) | 0.698 (0.04) | 0.14 |
| Cation–anion ratio (CAR) | 1.44 (0.07) | 1.45 (0.02) | 0.041a |
| Cations | 142.1 (4.7) | 145.01 (1.6) | 0.023a |
| Anions | 101.77 (7.09) | 102.09 (1.8) | 0.008a |
| Sodium–albumin ratio (SAR) | 258.49 (43.7) | 197.05 (11.3) | 0.04a |
| Osmolality–sodium ratio (OSR) | 2.12 (0.07) | 2.08 (0.05) | 0.66 |
| Osmolality–albumin ratio (OABR) | 547.3 (97) | 408.97 (26.8) | 0.036a |
| Osmolality–cations ratio (OCR) | 2.01 (0.06) | 1.96 (0.05) | 0.57 |
| Osmolality–anions ratio (OAR) | 2.86 (0.17) | 2.82 (0.09) | 0.097 |
Abbreviation: SD, standard deviation.
aSignificant at p value<0.05.
| Parameters | Disease severity score | |
|---|---|---|
| Pearson’s correlation (r) | p-Value | |
| Sodium | –0.089 | 0.332 |
| Chloride | –0.018 | 0.47 |
| Sodium–potassium ratio (SPR) | 0.069 | 0.37 |
| Sodium–chloride ratio (SCR) | –0.054 | 0.39 |
| Osmolality | –0.057 | 0.39 |
| Calcium | –0.367 | 0.033a |
| Phosphorus | –0.036 | 0.43 |
| Magnesium | –0.285 | 0.079 |
| Calcium–phosphorus ratio (CPR) | –0.025 | 0.45 |
| Calcium–magnesium ratio (CMR) | 0.094 | 0.32 |
| Albumin | –0.338 | 0.046a |
| Cation–anion ratio (CAR) | –0.066 | 0.37 |
| Cations | –0.129 | 0.265 |
| Anions | –0.026 | 0.45 |
| Sodium–albumin ratio (SAR) | 0.328 | 0.05a |
| Osmolality–sodium ratio (OSR) | 0.025 | 0.45 |
| Osmolality–albumin ratio (OABR) | 0.31 | 0.062 |
| Osmolality–cations ratio (OCR) | 0.055 | 0.39 |
| Osmolality–anions ratio (OAR) | –0.029 | 0.44 |
Note: r Pearson’s correlation coefficient.
aSignificant at p value<0.5.
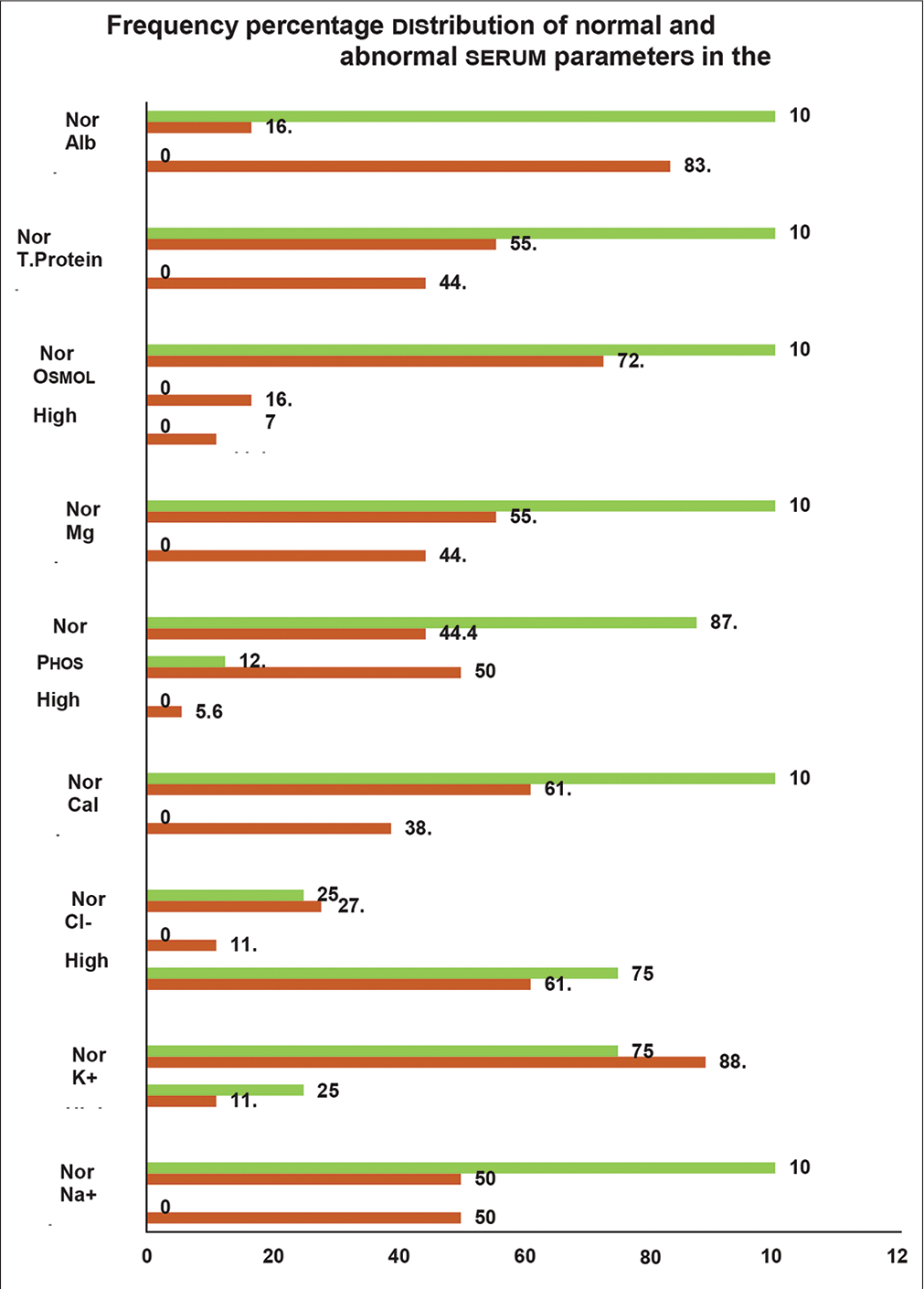
- Frequency percentage distribution of normal and abnormal serum parameters in the cases (N 26). mSignificant at p < 0.05; Na+, serum sodium; K+, serum potassium; Cl-, serum chloride; Cal, serum calcium; Phos, serum phosphorus; Mg, serum magnesium; Osmol, serum osmolality; T. protein, serum total protein; Alb, serum albumin.
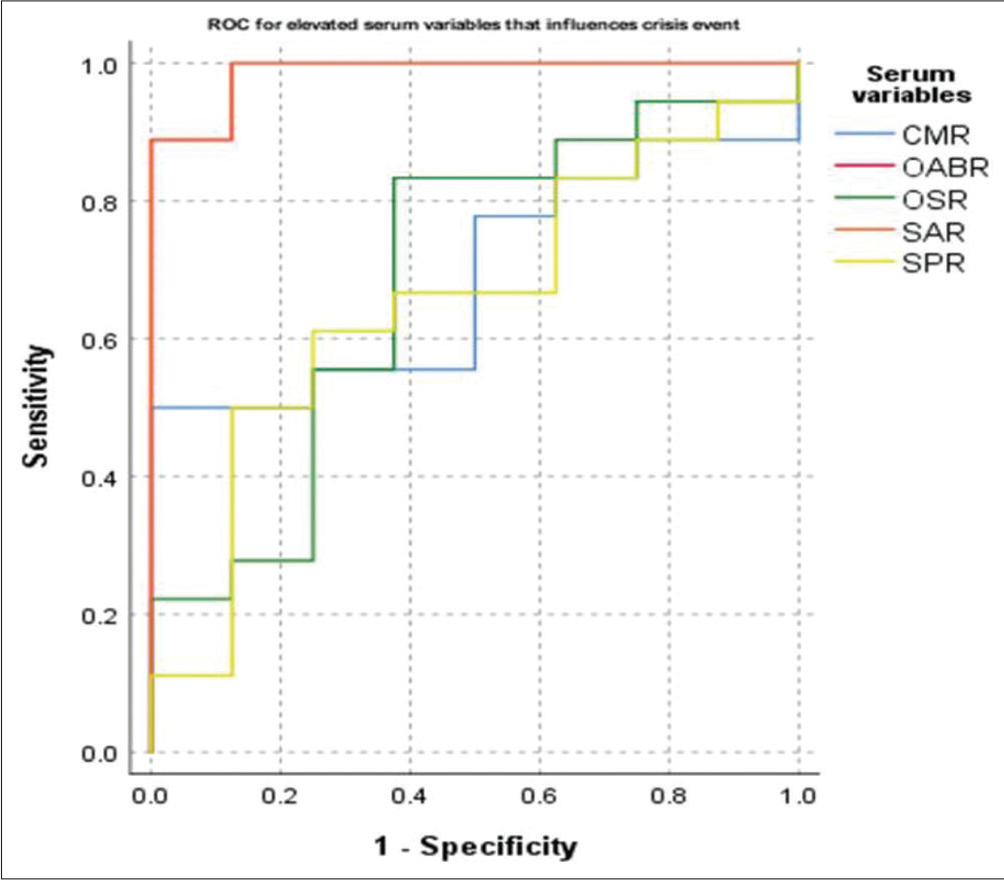
- Receiver operating characteristics (ROC) curve for elevated levels of the serum parameters that influence the crisis state to occur. CMR, calcium–magnesium ratio; OABR, osmolality– albumin ratio; OSR, osmolality–sodium ratio; SAR, sodium– albumin ratio; SPR, sodium–potassium ratio.
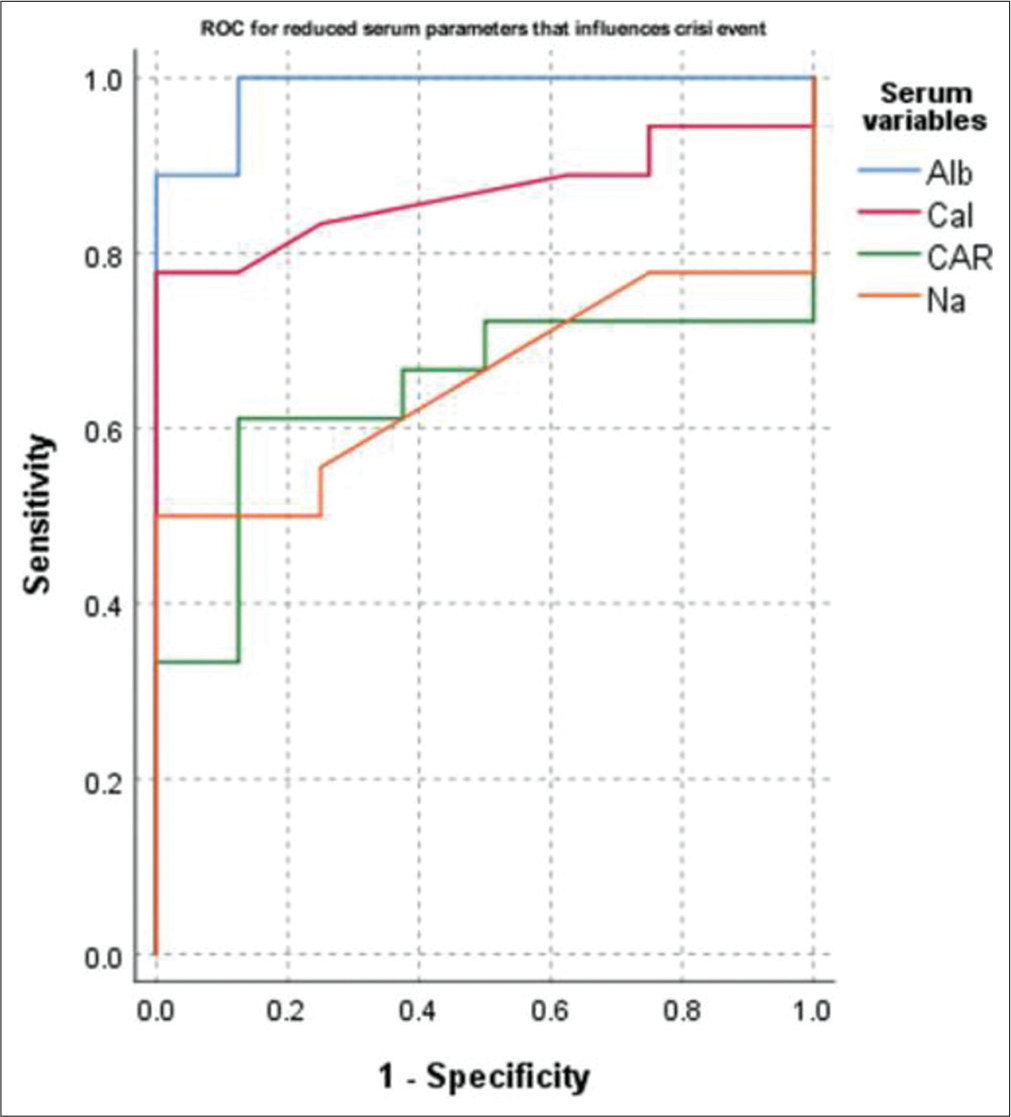
- Receiver operating characteristics (ROC) curve for reduced values of the serum parameters that influence the crisis state to occur. Na, serum sodium; Cal, serum calcium; Mg, serum magnesium; Alb, serum albumin; CAR, cation–anion ratio.
RESULTS
The study subjects comprised of 53 adult participants that included 26 cases of HbSS and 27 apparently healthy individuals as the control group. The mean (standard deviation), as depicted in Table 3, serum sodium (135.65 [4.17], p = 0.005), chloride (99.88 [5.9], p = 0.001), calcium (2.28 [0.2], p = 0.027), magnesium (0.76 [0.12], p = 0.004), total protein (7.46 [1.01], p < 0.001), and albumin (0.59 [0.11], p = 0.001) mmol/L, were lower in cases than the contemporary control group. In cases, serum osmolality was low (285.19 [9.15] mOsm/kg, p = 0.068). In addition, the overall cations (143.01 [4.2], p = 0.011) and anions (101.87 [5.9], p = 0.001) mmol/L, were also significantly decreased in cases, and accordingly, the ratios such as CMR, CAR, SAR, OABR, and OAR were elevated in cases.
The serum parameters were compared between the two groups of the cases, crisis state and steady state groups, as shown in Table 4. A significant reduction in serum sodium (135 [4.8] mmol/L, p = 0.01), chloride (99.8 [7.1] mmol/L, p = 0.008), calcium (2.21 [0.19] mmol/L, p = 0.011), CPR (1.63 [0.5], p = 0.049), cations (142.1 [4.7] mmol/L, p = 0.023), anions (101.77 [7.09] mmol/L, p = 0.008), and CAR (1.44 [0.07], p = 0.041) was observed in cases compared with crisis state. On the contrary, raised serum phosphorus (1.46 [0.36] mmol/L, p = 0.002), CMR (3.16 [0.5], p = 0.036), SAR (258.49 [43.7], p = 0.04), and OAR (547.3 [97], p = 0.036) were evidenced in crisis state individuals.
These parameters were correlated for disease severity score in cases as delineated in Table 5. The findings suggested serum calcium (r =–0.367, p = 0.033) and albumin (r =–0.338, p = 0.046) correlated inversely, whereas SAR (r = 0.328, p = 0.05) depicted a positive influence on disease severity score.
The AUC deciphered through ROC analysis for the occurrence of crisis events in cases are revealed in Table 6 and Figures 2 and 3. A gross AUC was observed for raised levels of SAR (AUC = 0.986, p < 0.001) and OABR (AUC = 0.986, p < 0.001; not shown). Similarly, the AUC was 0.868 for low serum levels of calcium (p = 0.003) and 0.986 for low serum albumin levels (p < 0.001).
| Parameters | AUC | p-Value | Cutoff valueb | Sensitivity | 1-Specificity |
|---|---|---|---|---|---|
| Sodium–albumin ratio (SAR) | 0.986 | < 0.001a | 208.45 | 0.944 | 0.125 |
| Osmolality–albumin ratio (OABR) | 0.986 | < 0.001a | 433.79 | 0.944 | 0.125 |
| Calcium (mmol/L) | 0.868 | 0.003a | 2.41 | 0.833 | 0.25 |
| Albumin (mmol/L) | 0.986 | < 0.001a | 0.63 | 0.889 | 0.125 |
Abbreviations: AUC, area under the curve; ROC, receiver operating characteristics.
aSignificant at p value<0.5.
bAs per kit inserts.
The cutoff values with 94.4% sensitivity for serum SAR and OABR were 208.45 and 433.79. The sensitivity for crisis events was 83.3 and 88.9%, respectively, for serum cutoff values of calcium and albumin at 2.41 and 0.63 mmol/L in cases.
The frequency percentage distribution of the normal and abnormal serum parameters in the cases is deciphered in Figure 1. Serum osmolality was found normal in all the steady state cases. However, in the crisis state group, low osmolality was noted in 11.1% and high osmolality in 16.7% of cases (chi-square = 2.751, p = 0.253). Abnormal serum osmolality would increase the probability of crisis state by nearly two times more than those with normal serum osmolality. The figure reflects that 50% of the cases presenting with a crisis state had low serum sodium, whereas none in the steady state had abnormal serum sodium values (chisquare = 4.89, p = 0.013). The OR was 11.59 (95% CI = 1.157– 389.7) for crisis events with low sodium than those within the normal reference range.
Similarly, low serum calcium and magnesium were noted in 38.9% (chi-square = 3.1, p = 0.039) and 44.4% (chi-square = 3.95, p = 0.023) of the crisis state cases, whereas none reported such lower values in steady state. The patients with low calcium were 7.52 times more prone to developing crisis episodes than those with normal serum values. On the contrary, raised serum phosphorus levels were recorded in 50% of the crisis state individuals compared with only 12.5% of steady state individuals (chi-square = 3.71, p = 0.03) with OR of 7.29 (95% CI = 0.87–197).
Of all the crisis state cases, 44.4% depicted low total protein and 83.3% represented low serum albumin (chisquare = 14.26, p < 0.001). None of the steady state cases had either low total protein or albumin. The chances for crisis were more than 50 times higher in individuals with low albumin than those with serum albumin within normal reference range (95% CI = 4.59–1864).
DISCUSSION
The case–control study included 27 apparently healthy adults and 26 HbSS SCD cases. The findings revealed a significant lowering of cations (p = 0.011) and anions (p = 0.001) in the cases than the control group [Table 3]. The serum osmolality was low in cases (p = 0.068), along with the significant extracellular ions contributing to serum osmolality, such as sodium and chloride. Antwi-Boasiako et al reported lower serum sodium and higher serum potassium in HbSS patients than in the control group (p < 0.001).[3] Similarly, other studies also reflected reduced serum sodium and raised serum potassium in SCD cases than the healthy control group.[16,17] Deoxygenation of the sickle cell RBCs is prone to altered cation permeability primarily attributed to red cell membrane Na+-K+ ATPase activity activation. Because of intracellular dehydration, the MCHC in sickle cell RBCs seems to be raised. The present study also observed a significant influence of serum osmolality with MCHC values (B = –0.110, p = 0.023; data not shown). The relative increase in HbS in these cells consequent to electrolyte imbalance results in rapid polymerization of the HbS. These events eventually result in vaso-occlusion in the microcirculation leading to crisis events and painful episodes. Brown et al study in a mouse model suggested that activated K+-Cl- cotransport worsens the disease pathogenesis and tissue damage.[5] Other articles also proposed that such abnormality could result in end-organ damage with increased morbidity like kidney dysfunction, cerebrovascular stroke, and mortality.[4,5,18] The results of the present study, low serum sodium and chloride, agreed with the above findings [Table 4] and propose that dehydration prevention would help reduce the crisis episodes. Besides serum electrolytes, serum calcium and magnesium were also grossly reduced in the cases of their healthy contemporary group [Table 3]. In addition, serum calcium depicted a significant negative correlation with disease severity [Table 5]. The explanation is that intracellular dehydration results from the activation of Gardos channels resulting in Ca2+ influx and loss of K+ and water. The net result is raised intracellular Ca2+ that triggers the downstream signals in the red cells, such as osmotic fragility, cytoskeleton instability, and membrane asymmetry for phospholipids distribution.[19,20] Magnesium, on the other hand, acts as a vasodilator and modulates the voltage-gated calcium channels. The resultant effect of hypocalcemia and hypomagnesemia is red cell dehydration and eryptosis (RBCs with features of senescence) followed by hemolysis and microvascular occlusions.[20] Consequent to hemolysis, serum phosphorus also tends to rise as depicted in this study. Previous studies also revealed that the CMR is high in SCD patients compared with healthy peer groups and could be used as an indicator for the extent of dehydration.[21,22] In agreement with the facts, the present study observed low serum calcium, magnesium, and raised phosphorus levels and accordingly low CPR and high CMR in the crisis group as compared with the steady state group [Table 4] Low serum calcium recorded a significant AUC as predictor for crisis state [Table 6] Serum calcium value less than 2.41 mmol/L (9.65 mg/dL) showed a sensitivity of 83.3% for crisis [Table 6 and Figure 3]. The observations suggested that these changes could be due to the shift of extracellular Ca2+ into the red cells during the crisis phase. Hence, treatment using calcium channel inhibitors and oral magnesium supplementation might be beneficial in preventing crisis episodes.
Serum albumin is generally considered a marker of the individual’s nutritional status. Besides, it is the primary determinant for the plasma oncotic pressure and, thus, the key determining factor for the fluid distribution between the extra- and intravascular compartments.[23] Low serum albumin levels correspond to low fluid holding capacity in the intravascular space, resulting in altered plasma osmotic pressure and osmolality. Plasma albumin is also attributed to RBC membrane stabilization and the shape and volume of RBCs.[8,24,25] Low albumin also depicted a significant inverse relation with the disease severity score [Table 5]. A cutoff value of 0.63 mmol/L (4.2 g/dL) showed a sensitivity of 88.9% for the crisis state [Table 6 and Figure 3]. Although the cations and anions in the crisis state were significantly decreased [Table 4], the ratios of serum osmolality with these variables were not different between the groups. Of all the ratios of osmolality with serum parameters, SAR and OABR reported a significant difference between the crisis and steady state groups [Table 4]. The sensitivity for a patient to have a crisis event was nearly 94.4% if the SAR values were higher than 208.45 [Table 6 and Figure 2]. Further, it was observed that an OAR value greater than 433.79 also depicted similar sensitivity of 94.4% (data not shown). Hence, the contribution of serum albumin toward fluid homeostasis and red cell membrane stability cannot be ignored. The findings agree with the Nouraie et al study, which observed a significant association of low serum albumin with higher mortality and proposed that albumin could be used as a biomarker for early assessment of clinical severity in SCD cases.[26] Furthermore, it was observed that in all the enrolled steady state cases [Figure 1] the levels of serum sodium, calcium, magnesium, osmolality, and albumin were within the normal reference range. None had low levels of these parameters. The key note of the present study is that prevention of red cell dehydration is one of the crucial factors for reducing the frequency of vaso-occlusive events. Hence, water and electrolyte balance should be considered for crisis prevention and management in these patients.
The study has some limitations. The small sample size is one of the significant limitations. A prospective longitudinal study including a larger cohort of HbSS cases that could be followed up regularly could provide a better understanding of the changes in serum ions and their influence on SCD pathophysiology. Instead of measuring total serum calcium and magnesium, ionized calcium and magnesium would have explored a more precise relationship with the disease severity in these patients. The study subjects’ nutritional status and total body water content were not assessed in this study, which could have provided better insight regarding the low serum albumin and magnesium. However, the advantage of the study is that it is a very comprehensive study that included the major serum cations and anions, including osmolality in the study groups, to elicit the prognostic impact of these parameters on disease severity. We could find very few studies that investigated all these serum variables in SCD cases. In addition, comparing the values with the healthy control group and between the crisis and steady state groups provided better insight into the changes occurring during the crisis. Further, additional research is necessary to determine the exact mechanism of the changes occurring in the microvasculature of SCD patients.
CONCLUSIONS
The present study confirmed that low serum sodium, calcium, and albumin were significantly associated with crisis events in SCD cases. In addition, raised SAR and OAR were also linked to the crisis state, and the resultant effects of the shift of ions between the compartments might be a key factor for intracellular dehydration and autopolymerization of HbS. Hence, routine estimation of these ions will help in the early assessment of the clinical severity. These parameters can be easily measured at any rural or urban health care center. Accordingly, SCD patients could be advised to proper fluid intake with adequate electrolyte and nutrient supplementations to maintain fluid homeostasis and prevent the frequency of crisis episodes.
HIGHLIGHTS
The major serum electrolytes and osmolality are reduced in SCD cases than their contemporary healthy individuals. Serum cations like sodium, calcium, and magnesium, and anions like chloride and albumin are decreased in the SCD crisis state resulting in disproportionate distribution of ions across the red cell membrane.
Low calcium, albumin, and sodium–albumin ratio are significantly associated with disease severity score and better sensitivity.
Routine estimation of these ions will help in the early assessment of clinical severity.
Adequate fluid and electrolyte intake would help maintain fluid homeostasis and prevent the frequency of crisis episodes in SCD cases.
Conflict of interest
None declared.
References
- Robbins & Cotran Pathologic Basis of Disease. Red Blood Cell and Bleeding Disorders. Vol 1. (10th ed). Elsevier; 2020. [Internet]. https://www.elsevier.com/books/robbins-and-cotran-pathologic-basis-of-disease/kumar/978-0-323-53113-9
- [Google Scholar]
- Pathophysiology of sickle cell disease. Annu Rev Pathol. 2019;14:263-292.
- [CrossRef] [PubMed] [Google Scholar]
- Serum potassium, sodium, and chloride levels in sickle cell disease patients and healthy controls: a case-control study at Korle-Bu Teaching Hospital, Accra. Biomark Insights. 2019;14:1177271919873889.
- [CrossRef] [PubMed] [Google Scholar]
- The clinical significance of K-Cl cotransport activity in red cells of patients with HbSC disease. Haematologica. 2015;100:595-600.
- [CrossRef] [PubMed] [Google Scholar]
- Activation of the erythroid K-Cl cotransporter Kcc1 enhances sickle cell disease pathology in a humanized mouse model. Blood. 2015;126:2863-2870.
- [CrossRef] [PubMed] [Google Scholar]
- Sickle cell dehydration: Pathophysiology and therapeutic applications. Clin Hemorheol Microcirc. 2018;68:187-204.
- [CrossRef] [PubMed] [Google Scholar]
- The function of ion channels and membrane potential in red blood cells: toward a systematic analysis of the erythroid channelome. Front Physiol. 2022;13:824478.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of osmolality and solutes on the morphology of red blood cells according to three-dimensional refractive index tomography. PLoS One. 2021;16:e0262106.
- [CrossRef] [PubMed] [Google Scholar]
- Extracellular fluid tonicity impacts sickle red blood cell deformability and adhesion. Blood. 2017;130:2654-2663.
- [CrossRef] [PubMed] [Google Scholar]
- The vaso-occlusive pain crisis in sickle cell disease: definition, pathophysiology, and management. Eur J Haematol. 2020;105:237-246.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of clinical severity of sickle cell anemia in Nigerian children. Journal of Applied Hematology. 2013;4:58-64.
- [Google Scholar]
- Relationship between disease severity and folate status of children with sickle cell anaemia in Enugu, South East Nigeria. Afr Health Sci. 2021;21:759-764.
- [CrossRef] [PubMed] [Google Scholar]
- Units of measure converter | AMA Manual of Style | Oxford Academic [Internet] https://academic.oup.com/amamanualofstyle/si-conversion-calculator
- [Google Scholar]
- Albumin Online converter from conventional units to SI units | UNITSLAB.COM [Internet] https://unitslab.com/node/31
- [Google Scholar]
- Converters and Calculaters for all your needs [Internet] cm2feet.com. https://cm2feet.com/
- [Google Scholar]
- Haematological indices & electrolyte status in sickle cell disease at rural hospital of central Maharashtra. In: Int J Med Sci Public Health. Vol 3. 2014. p. :1410-1412.
- [CrossRef] [Google Scholar]
- Biochemical indicator of sickle cell disease: preliminary report from India. In: Indian J Clin Biochem. Vol 27. 2012. p. :191-195.
- [CrossRef] [PubMed] [Google Scholar]
- The erythroid K-Cl cotransport inhibitor [(dihydroindenyl)oxy]acetic acid blocks erythroid Ca2+ activated K+ channel KCNN4. Am J Physiol Cell Physiol. 2022;323:C694-C705.
- [CrossRef] [PubMed] [Google Scholar]
- Ion transport pathology in the mechanism of sickle cell dehydration. Physiol Rev. 2005;85:179-200.
- [CrossRef] [PubMed] [Google Scholar]
- Is increased intracellular calcium in red blood cells a common component in the molecular mechanism causing anemia? Front Physiol. 2017;8:673.
- [CrossRef] [PubMed] [Google Scholar]
- Total serum magnesium levels and calcium-to-magnesium ratio in sickle cell disease. Medicina (Kaunas). 2019;55:547.
- [CrossRef] [PubMed] [Google Scholar]
- Red blood cell and serum magnesium levels among children and adolescents with sickle cell anemia. Biol Trace Elem Res. 2018;186:295-304.
- [CrossRef] [PubMed] [Google Scholar]
- Physiology, Water Balance [Updated October 3, 2022] In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023.
- [Google Scholar]
- Albumin reverses the echinocytic shape transformation of stored erythrocytes. Clin Hemorheol Microcirc. 2015;60:437-449.
- [CrossRef] [PubMed] [Google Scholar]
- Washing stored red blood cells in an albumin solution improves their morphologic and hemorheologic properties. Transfusion. 2015;55:1872-1881.
- [CrossRef] [PubMed] [Google Scholar]
- Serum albumin is independently associated with higher mortality in adult sickle cell patients: results of three independent cohorts. PLoS One. 2020;15:e0237543.
- [CrossRef] [PubMed] [Google Scholar]





