Translate this page into:
Bloodstream Infections with Opportunistic Pathogens in COVID-19 Era: A Real Challenge Necessitates Stringent Infection Control
Address for correspondence: Bhaskar Narayan Chaudhuri, MD, Department of Microbiology, Peerless Hospitex Hospital and Research Center Ltd., Kolkata-700094, West Bengal, India (e-mail: bhaskarnchau@gmail.com).
-
Received: ,
Accepted: ,
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Bloodstream infections (BSI) due to opportunistic microbes in the coronavirus disease 2019 (COVID-19) pandemic lead to high morbidity and mortality among hospitalized patients. Thus, it is vital to find out the risk factors of BSI and to learn the ways to mitigate it.
Aim:
The aim of this study was to evaluate important risk factors of BSI due to opportunistic pathogens and to assess the role of the rigid infection control program to deal with this issue.
Methods:
A prospective, cross-sectional study was performed for 6 months on 150 patients admitted in both COVID-19 and non-COVID-19 intensive care units of our hospital. BSI was confirmed by the BACTEC and Vitek 2 compact system. Prospective surveillance and environmental sampling were carried out for source tracking along with rigorous infection control measures and the outcome was analyzed.
Findings:
Burkholderia cepacia, Elizabethkingia meningoseptica, Candida auris, vancomycin-resistant Enterococcus, and Achromobacter xylosoxidans were the common opportunistic pathogens isolated from a single or paired blood sample(s) in our study. Key risk factors were prolonged intensive care unit stay, central venous access, mechanical ventilation, immune-compromised condition, and use of biologics. Reverse osmosis water and used normal saline bottles were the common environmental source of infection. Following the implementation of precise infection control measures, there was a sharp decline in BSI cases, which was not attributed to the downfall of COVID-19 cases.
Conclusion:
Combined prospective surveillance and environmental sampling helped to find out the sources and implementation of an intensive and insistent infection control program that are needed to control opportunistic pathogens mediated BSI.
Keywords
opportunistic pathogens
Burkholderia cepacia
Elizabethkingia meningoseptica
non-albicans Candida
bloodstream infection
infection prevention and control
Introduction
Bacterial and fungal bloodstream infection (BSI) during intensive care unit (ICU) stay has been reported in other outbreaks of severe acute respiratory syndrome (SARS), but there is limited data on BSI by opportunistic infections available in the context of SARS-CoV-2 pandemic.[1] Many authors have recognized the importance of superinfection, but definitive data are still lacking.
The role of the host response to infection by SARS-CoV-2 in coronavirus disease 2019 (COVID-19) disease has been used as a potential target for therapy, and several immunomodulatory treatments have been proposed throughout the outbreak.[2] Steroid therapy use, with varying doses and regimens, and the use of specific biologic drugs such as tocilizumab may lead to profound immune suppression,[3] and use of broad-spectrum antibiotics and multiple antibiotics, sometimes for a prolonged period, may lead to the emergence of drug-resistant pathogens.[4] On the other hand, inadequate infection prevention and control (IPC) measures, including improper hand hygiene before and in between patients contact, lack of bundle care while dealing intravenous (IV) lines, and deployment of new staff without adequate training, may be attributed to the emergence of drug-resistant strains and cross-infections among COVID-19 patients admitted in ICU and non-ICU wards.[5,6]
Materials and Methods
This was a prospective, cross-sectional study conducted in the Department of Microbiology, Peerless Hospitex Hospital and Research Center Ltd. for a period of 6 months, spanning from September 2020 to February 2021. A total of 150 patients admitted in both COVID-19 and non-COVID-19 Intensive therapy units, ICU, and non-ICU wards were enrolled in the study. The study obtained permission from the institutional ethical committee, vide letter number—PHH and RCL/CREC/FM02 dated 9th September 2021. The relevant demographic characteristics and risk factors were assessed and analyzed, including age, gender, duration of hospitalization, presence of prior antibiotic treatment, clinical comorbidities, duration of exposure to central venous access, device days, history of indwelling devices, and clinical outcome. Laboratory testing for infection control was mainly targeted at three major areas—antibiotic susceptibility testing, detection and identification of infectious disease (ID) pathogens, and finally, genotyping methods. Stringent strategies have been implemented to prevent and control the spread of multidrug-resistance organisms (MDROs) like carbapenem-resistant Enterobacteriaceae (CRE), Multidrug-resistant Acinetobacter & Pseudomonas species, Methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus (VRE), etc. and to minimize the risk of cross-infection to other patients, staff and visitors. For rapid identification of patients or environmental sources colonized with these MDROs, active surveillance (like CRE, VRE, and MRSA surveillance) was carried out from rectal swabs, feces, nostrils, groin, and armpits for high-risk patients admitted in ICUs, as per Centers for Disease Control and Prevention (CDC) guidelines. For timely management of patients, the detection of antimicrobial drug-resistant genes was carried out by different phenotypic (EDTA Carbapenem inactivation method, Modified Carbapenem inactivation method) and genotypic methods like real-time polymerase chain reaction (RT-PCR). Non-molecular-based approaches like Enzyme-linked fluorescence assay to detect C. difficile toxin in stool, rapid immunochromatographic card tests, and immune- blot assays were also used.
Inclusion Criteria
All patients aged 18 years and above with a positive SARS-CoV-2 RT-PCR result and positive blood culture for opportunistic bacterial or fungal pathogens on one occasion or a pair of blood cultures revealing the growth of the same opportunistic pathogen were included and reviewed by an ID specialist to determine the presence of true clinical co-infection and the source.
All adult patients admitted to ICU, who presented with fever or other site-specific infection due to infective etiology with BSI, 48 hours following hospital admission satisfying healthcare associated infection (HAI) criteria, were included in the study.
A National Healthcare Safety Network criterion was used for central-line-associated BSIs and catheter-related BSIs.
Patient-specific nonduplicate isolates were included for analysis.
Exclusion Criteria
Patients who developed BSI within 48 hours of hospital admission, previous hospital admission in the past 1 week, immune deficient patients, patients with suspected viral or parasitic infection or autoimmune disease, and patients with radiological evidence of active tuberculosis or cancer were excluded from the study.
Microbiological Investigation
Laboratory diagnosis of sepsis was confirmed by collecting a set of two blood samples. Blood was collected in BD BACTEC plus aerobic blood culture bottles, incubated, and monitored regularly using the BD BACTEC FX40 Blood culture system (BD Diagnostic, BD HEAD OFFICE, 6th Floor Signature Tower - B South City I, NH 8, Gurgaon, 122001, Haryana India, Tel : 0124-4124300, Fax: 91-124-2383224-25-26, E-mail: bd_india@bd.com). All bottles with positive signals were removed from the instrument, Gram-stained, and subcultured on blood agar and MacConkey agar. Phenotypic identification was confirmed with Vitek 2 ID-GNB/GP/YST cards (bioMérieux, 43A, near Modi Mill Compound, Okhla Phase III, Okhla Industrial Estate, New Delhi, Delhi 110020 India). If multiple blood samples sent within 48 hours duration from the same patient yielded the same isolate with an identical antibiogram, it was considered a single (nonduplicate) isolate. Antimicrobial susceptibility of the clinical isolates was determined both by disc diffusion method and automated susceptibility using Vitek 2 AST N 363, 364; P628 and YS08 card (bioMérieux, India).
Environmental and Personnel Specimen Cultures:
Combined prospective surveillance and environmental sampling were performed for source tracking. Microbiological cultures were first obtained from thumb-prints (on culture media) of the healthcare staff/ doctors, axilla and groin swabs from suspected cases to rule out Candida auris colonization (as per CDC guidelines), and rectal swabs from suspected cases with CRE and VRE carriage. No significant findings were obtained from those samples except for C. auris isolated from the groin swab of a patient, and rectal swab culture showing growth of VRE from two patients. Subsequently, we focused on collecting swabs from environmental surfaces of different wards: normal saline used for ITU/ICU/non-ICU patients; 50 mL syringe with IV preparation; common normal saline bottles from nursing stations; reverse osmosis (RO) water (kept in jars) used as humidifier; used Cidex OPA solution; tap water, shower water; alcohol-based hand rub, disinfectants; dialysis unit RO inlet and outlet water.
A total of 105 environmental specimens were collected and cultured on Chrome agar and MacConkey agar. Organisms, if grown, were identified subsequently using the Vitek-2 compact system.
Infection Control Investigation:
Intensive and aggressive infection control measures were taken, including cleaning the ICU and fumigation of the ICU using hydrogen peroxide vapor and water disinfection. The use of sterile gloves and barrier nursing was ensured; closed monitoring of the reconstitution and preparation of IV medication was done. Apart from these, most importantly hand hygiene compliance among health care workers (HCWs) in our ICUs was assessed. The adequacy of general environmental cleaning and disinfection of reusable medical equipment were also evaluated. Bundle care for both central and peripheral intravenous lines including maintenance of strict asepsis during central vascular catheter (CVC) insertion, line care/manipulation including flushing line with 10 mL sterile water, and medication administration were observed for the identification of possible infection control lapses during patient care. Besides, thorough monitoring and tracking of different HAIs rates, compliance to Bundle care, and biomedical waste management were done.
As a cluster of the organism isolated from different clinical samples shared the same phenotypic characteristics and susceptibility profile, an extensive study of the suspected outbreak was conducted from time to time with proper case definition, selection of specimens for study, and steps were taken following the outcome analysis. Local antibiogram data of ICU and non-ICU settings were also generated every 2 months and the antibiotic policy of the hospital was re-evaluated yearly to monitor abnormal changes in the trend of infections.
Statistical Analysis
All graphs were generated in MS Excel. The statistical analyses used were the two-sample test for equality of proportions (chi-square test), and test for the Pearson's correlation coefficient. A p-value of less than 0.05 was considered significant.
Results
Out of the total of 150 patients admitted during the study period, 54 patients with suspected clinical sepsis developed a laboratory-confirmed bloodstream infection (LCBI) on one or more occasions. A total of 54 opportunistic nonduplicate isolates were detected from 54 LCBI patients over 6 months from September 2020 to February 2021. The study population constituted 44 (81.48%) males and 10 (18.5 %) females. The median age of infected patients was 62 years (interquartile range: 52.5–72). The mean length of hospital stay was 8 ± 1 day (range: 3–62 days) and the mean length of ICU stay was 6.4 ± 2 days (range: 3–31 days).
In total, 125 (83.3%) out of 150 patients were admitted to intensive care units (ITU and ICU), and the rest to nonintensive care wards. Various risk factors were observed among 54 patients with BSI caused by opportunistic pathogens, including a prolonged length of hospital stay of more than 7 days, central venous access, and exposure to mechanical ventilation for more than 5 days among others, in comparison to the noninfected cases (n = 96). Risk factors for cases and matched controls are listed in ►Table 1.
| Risk variables | Cases (n = 54) | Controls (n = 96) | p-Value |
|---|---|---|---|
| Prolonged ICU stay >7 days | 41 (75.9%) | 57 (59%) | 0.0378c |
| Central venous access | 46 (85.2%) | 62 (64.6%) | 0.0072c |
| Exposure to Mechanical ventilation for > 5 days | 43 (79.6%) | 59 (61.5%) | 0.0230c |
| Age >60 years | 24 (44.4%) | 30 (31.2%) | 0.1070 |
| Neutropenia | 5 (9.3%) | 21(21.9%) | 0.0513c |
| Immunocompromiseda | 34 (62.9%) | 43(44.8%) | 0.0339c |
| Diabetes mellitus | 14 (25.92%) | 19 (19.8%) | 0.3867 |
| Biologicsb | 3 (5.5 %) | 2(0.02%) | 0.0216c |
| Steroids use | 7 (12.9%) | 18(18.8%) | 0.3537 |
Abbreviations: HIV, human immunodeficiency virus; ICU, intensive care unit; IL-6, interleukin 6.
a Immunocompromised includes chronic diabetes, HIV, hepatitis C, active malignancy, organ transplant, rheumatologic disease, or chronic receipt of immunosuppressive medications.
b Tocilizumab (antagonist of the IL-6 receptor) and similar agents.
c p-Value of the differences is significant at 0.05 level.
Microbiological Workup
A total of 54 nonduplicate clinical isolates were obtained from blood samples of these patients admitted to ITU, ICU, and non-ICU general wards. The mean time from admission to culture positivity was 8.5 days. The incidence of opportunistic pathogens causing BSI is depicted in ►Table 2.
| Pathogens | Sept 2020 | Oct 2020 | Nov 2020 | Dec 2020 | Jan 2021 | Feb 2021 | Total (%) |
|---|---|---|---|---|---|---|---|
| Burkholderia cepacia | 0 | 0 | 2 | 4 | 7 | 0 | 13 (24.1) |
| Elizabethkingia meningoseptica | 1 | 2 | 3 | 3 | 1 | 0 | 10 (18.5) |
| Candida auris | 1 | 2 | 2 | 1 | 0 | 1 | 7 (12.9) |
| VRE | 1 | 0 | 3 | 1 | 0 | 0 | 5 (9.2) |
| Achromobacter xylosoxidans | 0 | 1 | 1 | 0 | 0 | 1 | 3 (5.5) |
| Candida parapsilosis | 0 | 1 | 1 | 1 | 0 | 0 | 3 (5.5) |
| Candida guilliermondii | 1 | 0 | 1 | 0 | 0 | 0 | 2 (3.7) |
| Chryseobacterium indologenes | 0 | 1 | 1 | 0 | 0 | 0 | 2 (3.7) |
| Stenotrophomonas maltophilia | 0 | 1 | 0 | 1 | 0 | 1 | 3 (5.5) |
| Aeromonas hydrophilia | 0 | 1 | 1 | 0 | 0 | 0 | 2 (3.7) |
| Candida glabrata | 1 | 0 | 0 | 0 | 0 | 0 | 1 (1.85) |
| Candida haemulonii | 1 | 0 | 0 | 0 | 0 | 0 | 1 (1.85) |
| Aeromonas sobria | 0 | 1 | 0 | 0 | 0 | 0 | 1 (1.85) |
| Alcaligenes faecalis | 0 | 0 | 0 | 1 | 0 | 0 | 1 (1.85) |
| Total number of opportunistic pathogens causing BSI | 54 | ||||||
Abbreviation: BSI, bloodstream infection.
Out of 54 BSI isolates, Burkholderia cepacia topped the list accounting for 13 cases, followed by Elizabethkingia meningoseptica that accounted for 10 cases. The third important group was the non-albicans Candida species, of which C. auris isolates were recovered from seven patients. Remaining were shared among VRE and other nonfermenting gram-negative bacilli (Chryseobacterium indologenes; Aeromonas species; Stenotrophomonas maltophilia, and Alcaligenes faecalis). All clinical isolates obtained from the same genus showed similar morphological appearance and growth characteristics on microbial culture.
After a thorough root cause analysis, we concluded that such occurrence could probably be due to improper hand hygiene, improper use of gloves between patients, prolonged storage of RO water in jars for more than 48 hours that could have helped waterborne pathogens to grow, common NS being used for inotrope preparation, improper bundle care related to intravenous lines, more incidence of femoral lines, especially in COVID-19 patients, and deployment of new nursing staff without adequate training of IPC measures.
Antimicrobial susceptibility testing revealed that all the isolates under the same genus exhibited a similar antibiogram.
As a part of intensive source tracking and control, we screened a total of 105 environmental samples at periodic intervals. Three used normal saline samples from ICU patients grew nonlactose fermenting, late oxidase-positive, and gram-negative bacilli, which were subsequently identified as B. cepacia by Vitek 2 system.
Three RO water samples in a jar (used for a humidifier)—one each from ITU, ICU, and Netaji ward also showed growth of B. cepacia. All six isolates from environmental sources showed similar growth characteristics with that of clinical isolates and shared a similar antibiogram. In total, we could identify 17 isolates out of 105 environmental samples tested. Details of the isolates from surveillance samples are summarized in ►Table 3.
| Environmental samples | From ITU (No. of samples) | From ICU (No. of samples) | From Non-ICU Wards (Netaji, Deshbandhu, BC Roy, KTU, etc.) (No. of samples) | Identified organisms by Vitek 2 Compact System, their numbers and sources |
|---|---|---|---|---|
| Used NS for patients | 2 | 3 | 2 | Burkholderia cepacia: 3 (all from ICU) |
| 50 mL syringe with IV preparation | 1 | 1 | 1 | 0 |
| Common NS from nursing station | 1 | 1 | 1 | Acinetobacter baumannii: 1(from ICU) |
| RO water used for humidifier | 2 | 2 | 8 |
B. cepacia: 3 (one each from ITU, ICCU and Netaji) P. aeruginosa: 1 (from ITU) A. baumannii: 1 (from ICU) |
| Intact saline | 1 | 1 | 4 | 0 |
| Tap water | 1 | 1 | 3 |
A. baumannii:2 (one each from ITU and ICU) Dual growth of Aeromonas hydrophila and Sphingomonas paucimobilis: 2 (one each from Netaji and DB ward) |
| Shower water | 0 | 0 | 3 | Acinetobacter lwoffii: 1 (from Tagore/ Executive ward) |
| Glutaraldehyde (Cidex) OPA solution | 1 | 1 | 0 | Escherichia coli: 1 (from ICU) |
| Alcohol based hand rub | 1 | 1 | 5 | 0 |
| Dialysis unit RO inlet water | 1 | 1 | 19 | 0 |
| Dialysis unit RO outlet water | 0 | 0 | 12 | 0 |
| Dialysis unit wash area water | 0 | 0 | 12 | 0 |
| Dialysis unit machine inlet water | 0 | 0 | 12 | 0 |
| Total surveillance sample = 105 | 17 isolates grown | |||
Abbreviations: ICU, intensive care unit; ITU, intensive therapy unit; NS, normal saline; OPA, ortho phthalaldehyde; RO, reverse osmosis.
The mortality rate was alarmingly high among patients with BSI caused by opportunistic pathogens, especially with Elizabethkingia meningoseptica, Stenotrophomonas maltophilia, B. cepacia, and Candida guilliermondii. Details of mortality analysis are depicted in ►Table 4.
| Name of the opportunistic pathogens | Number of cases | Expired patients (n) | Mortality rate (%) | ICU | Non-ICU |
|---|---|---|---|---|---|
| Elizabethkingia meningoseptica | 15 | Expired (12) | 80% | 10 (1 neurology NNC ITU) | 5 |
| Burkholderia cepacia | 20 | Expired (8) | 44% | 19 (2 neurology NNC ITU) | 1 |
| Stenotrophomonas maltophilia | 2 | Expired (2) | 100% | 2 | 0 |
| Candida guilliermondii | 3 | Expired (2) | 66.7% | 3 | 0 |
Abbreviations: ICU, intensive care unit; ITU, intensive therapy unit; NNC, National Neuroscience of Calcutta.
After a few consecutive cases, detailed systematic and rigorous infection control practices were initiated to prevent the further spread of these opportunistic bugs. Initially, the infected patients were isolated and cohorted using physical barriers within the cubicle. Every cubicle was disinfected by fogging using Silvicide (0.01% w/v silver nitrate and 10% w/v hydrogen peroxide) vapor. Intensive terminal cleaning of cubicles, bed rails, and bedside objects was done with Virex 256 (quaternary ammonium compounds). Thorough cleaning of the curtains, bedding, and linen was done using appropriate heat disinfection with hospital-graded detergents. Strict hand hygiene with plain or antimicrobial soap and water or alcohol-based hand rub was reinforced. Enhanced barrier precautions, that is, the use of personal protective equipment was encouraged if contact with patients or objects surrounding patients was anticipated, and dedicated instruments (e.g., stethoscope/blood pressure cuff) was also allocated, as it has been seen to disrupt the spread of MDROs among the patients with devices. Rational use of gloves and interchanging of gloves between two patients while handling lines and suctioning was strictly followed by doctors, nurses, and paramedics. Infection control nurses were vigilant to monitor the same meticulously. The unnecessary use of gloves was discouraged in non-COVID-19 wards. A thorough training was given to all HCWs, especially in COVID-19 wards, to ensure bundle care of IV lines. The common use of saline bottles for flushing and preparation of inotrope and other medications was strictly prohibited. It was decided to use 100 mL normal saline bottles for each patient individually for preparation of medication and flushing, etc. by adhering to infection control practices. Reuse was restricted to the same patient and no syringe was to be reused (for the same patient) for more than 24 hours. All IV fluids, TPN, and medications were scrupulously checked and administered with significant caution. Feed preparation was strictly monitored. Thorough cleaning of water tanks with pipelines and chlorination was immediately done and then regularly monitored at regular intervals.
The hand hygiene compliance rate was measured on monthly basis. An increase in hand hygiene compliance from 79.8 to 87.8 % was observed over 6 months from September 2020 to February 2021. Central line insertion was closely monitored, and maintenance was regularly assessed and recorded in a standard proforma as per CDC guidelines. Central line bundle care compliance was assessed and recorded daily and special training was given to HCWs to increase compliance with aseptic procedures and techniques. There was an increase in central line bundle care compliance from the baseline rate of 75.6 to 89.8 % observed over 6 months from September 2020 to February 2021 (►Fig. 1).

- Improved hand hygiene compliance and central vascular catheter (CVC) bundle care compliance (in percentages) after thorough training.
All concerned staff and HCWs participated in infection control meetings held regularly, to discuss measures and recommendations to control this unusual surge of BSI by opportunistic pathogens. After assiduous, exhaustive, and timely efforts along with efficient source tracking and control activities, a drastic reduction in cases of BSI by opportunistic pathogens was observed over the next couple of months, and cases with isolation of these characteristic strains reduced to near zero after March 2021 (►Fig. 2).

- Decrease in bloodstream infection (BSI) by opportunistic pathogens following implementation of infection prevention and control (IPC) measures. Y axis indicates number of isolated opportunistic pathogens. ICU, intensive care unit; VRE, vancomycin-resistant Enterococcus.
There was a gradually decreasing trend of COVID-19 cases in the city at the end of the study period. Thus, to find out a correlation between a decrease in opportunistic pathogen-mediated BSI and decreasing number of COVID-19 cases in the city, we performed a statistical analysis, which revealed that decreased opportunistic BSIs were not attributed to decreasing trend of COVID-19 cases in the city (►Fig. 3). Pearson correlation coefficient between the month-wise number of opportunistic blood-borne pathogens and total active COVID-19 cases reported in Kolkata were calculated. The calculated R was 0.7356. However, the p-value was found to be 0.095619. The result was not significant at 0.05 levels. Thus, it indicates that no relationship was present between the two variables.
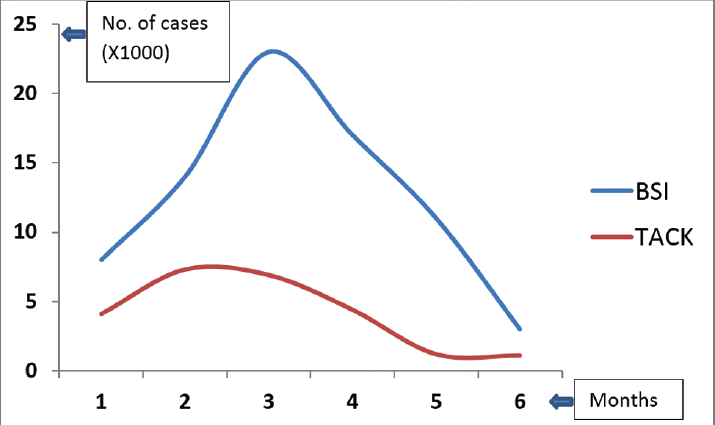
- Graph showing number of bloodstream infection (BSI) and total number of coronavirus disease 2019 active cases (x1000) in Kolkata (TACK), X-Axis: 1-Sep, 2020, 2-Oct, 2020, 3-Nov, 2020, 4-Dec, 2020,5-Jan, 2021, 6-Feb, 2021.
Discussion
In recent times, opportunistic bacterial and fungal pathogens, such as B. cepacia, Elizabethkingia meningoseptica, other nonfermenting gram-negative bacilli, and Candida non-albicans species are being detected in the bloodstream with increased frequency, especially from COVID-19 patients.[7,8]
Nonfermenting gram-negative bacilli like B. cepacia, Elizabethkingia meningoseptica, and Stenotrophomonas maltophilia are primarily environmental bacteria residing mainly in water and aqueous solutions.[9] They cause opportunistic infections in immune-compromised subjects including those on prolonged broad-spectrum antibiotic and steroid therapy. Although they may be transmitted from one patient to another through improperly disinfected hands of caregivers and aerosols, those are not the common routes of spread. The organisms have to be introduced into the bloodstream through contaminated syringes or from IV hubs to colonize the IV lines or cause BSIs.[10]
In our study, prolonged ICU stay, central venous access, exposure to mechanical ventilation, immune-compromised condition, and use of biologics were found to be significant risk factors of BSI, which corresponds to observations of other workers.[11-13] Although higher age, diabetes mellitus, and steroid use appear as important risk factors for BSI, in this study we did not find any significant difference with the control population. Paradoxically, neutropenia appeared to be a significant risk factor, predominantly seen with control as compared to cases (29.3% in control vs 9.3% in cases).
B. cepacia (24.1%) was most commonly isolated followed by E. meningoseptica (18.5%); C. auris (12.9%), VRE (9.2%), and A. xylosoxidans (5.5%) among many other opportunistic pathogens in our study. B. cepacia is a well-reported pathogen known to be associated with many hospital outbreaks, attributed to a plethora of sources: antiseptics, disinfectants, and nebulizer solutions[14] (Memish et al., 2009). In our study, we were able to isolate B. cepacia from six environmental samples, three each from used saline bottles and from RO water that was kept in a jar to be used as a humidifier. Various studies have shown that E. meningoseptica is capable to survive in chlorinated water, disinfectants, the contaminated hand of HCWs, infant formulas, and often colonizing sinks, taps, etc. Contaminated medical devices involving saline solution used for flushing, ventilators, breathing circuits, endotracheal tubes, humidifiers, and intravascular devices have been documented as of serious concern and creating potential reservoirs for healthcare-associated infections.[14,15] A study conducted by Khan et al showed that the administration of broad-spectrum antibiotics including Colistin was found to be a predisposing factor to cause invasive infection by E. meningoseptica.[16] In our study, 7 out of 10 patients (70%) suffering from E. meningoseptica bacteremia had a previous history of broad-spectrum antibiotics exposure including Colistin. C. auris is an emerging fungus that can cause invasive infections associated with high mortality and is often resistant to azoles, Amphotericin B, and Flucytosine and sensitive to Echinocandins only. It often colonizes the axilla and groin of hospitalized patients before it can result in invasive infection in hosts. We were able to identify one isolate of C. auris from a groin swab collected from a patient during active surveillance screening in our hospital as per CDC guidelines. In our study, BSI caused by A. xylosoxidans was reported only in three cases, which is similar to the study conducted by Houlihan et al, where it had been reported rarely.[16] In this study, we also observed no significant correlation between opportunistic BSI and the total number of active COVID-19 cases in our locality.
Most of the opportunistic pathogens isolated from blood were also recovered from different water sources used in the hospital for various purposes. Thus, water contamination is an important factor for these opportunistic BSI. This finding corresponds to the findings of other workers.[17] A high mortality rate was observed in BSI by opportunistic bugs like E. meningoseptica (80%) and B. cepacia (44%) in our study, as also observed by other authors.[18] Both hand hygiene compliance (from 79.8 to 87.8 %) and CVC bundle care compliance (from 75.6 to 89.8 %) had improved significantly after thorough training (►Fig. 1), which is similar to the study conducted by Rastogi et al[19] and these were probably reflected in curbing the rate of BSI following IPC training. In agreement with other studies, coordinated infection control activities such as implementing standard hand hygiene, barrier and contact precautions, cohorting of patients having the same opportunistic infections, environmental surveillance and source tracking, water disinfection, and enhanced and terminal cleaning of the ICUs helped us to decrease BSI rates significantly over next couple of months (March and April 2021) in our hospital (►Fig. 2).
Clonal relatedness could not be established for environmental isolates with that of clinical isolates, due to the lack of multilocus sequence testing facility in our hospital.
Conclusions
BSI due to opportunistic microbes is seen to be on the rise during the COVID-19 pandemic. B. cepacia, E. meningoseptica, C. auris, VRE, and A. xylosoxidans are the common opportunistic pathogens isolated from BSI in our study. Common sources were identified as RO water and used normal saline bottles. Early diagnosis and appropriate therapy, along with intensive and persistent infection control practices, are required for the successful containment of these pathogens and to curb such clusters of opportunistic infections.
Acknowledgement
The authors acknowledge the support of the administration section of the Peerless Hospitex Hospital and Research Center Ltd., particularly Dr. S. K. Purkayastha, Managing Director and Dr. Subhrojyoti Bhowmick, Academic Director for their continuous support in our research programs.
Conflict of Interest
None declared.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Opportunistic infections in COVID-19: a systematic review and meta-analysis. Cureus. 2022;14(03):e23687.
- [CrossRef] [Google Scholar]
- Immunomodulatory drugs in the management of SARS-CoV-2. Front Immunol. 2020;11:1844.
- [CrossRef] [PubMed] [Google Scholar]
- Tocilizumab in severe COVID-19 pneumonia and concomitant cytokine release syndrome. Eur J Case Rep Intern Med. 2020;7(05):001675.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 and antimicrobial resistance: a cross-study. Sci Total Environ. 2022;807(Pt 2):150873.
- [CrossRef] [PubMed] [Google Scholar]
- Infection prevention and control during COVID-19 pandemic: realities from health care workers in a north central state in Nigeria. Epidemiol Infect. 2021;149:e15.
- [CrossRef] [PubMed] [Google Scholar]
- Antimicrobial resistance and COVID-19: Intersections and implications. eLife. 2021;10:e64139.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19-associated opportunistic infections: a snapshot on the current reports. Clin Exp Med. 2022;22(03):327-334.
- [CrossRef] [PubMed] [Google Scholar]
- Secondary bacterial infection in COVID-19 patients is a stronger predictor for death compared to influenza patients. Sci Rep. 2021;11(01):12703.
- [CrossRef] [PubMed] [Google Scholar]
- Waterborne outbreaks in hemodialysis patients and infection prevention. Open Forum Infect Dis. 2022;9(03):ofac058.
- [CrossRef] [PubMed] [Google Scholar]
- Bloodstream Infection Event (Central Line-Associated Bloodstream Infection and Non-central Line Associated Bloodstream Infection), National Healthcare Safety Network, CDC, Atlanta, January. 2023
- [Google Scholar]
- Central Line Associated Blood Stream Infections. In: In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. Accessed January 24, 2023 at: https://www.ncbi.nlm.nih.gov/books/NBK430891/
- [Google Scholar]
- COVID-19 increased the risk of ICU-acquired bloodstream infections: a case-cohort study from the multicentric OUTCOMEREA network. Intensive Care Med. 2021;47(02):180-187.
- [CrossRef] [PubMed] [Google Scholar]
- Acinetobacter baumannii isolates from COVID-19 patients in a hospital intensive care unit: molecular typing and risk factors. Microorganisms. 2022;10(04):722.
- [CrossRef] [PubMed] [Google Scholar]
- Saudi National Guard Infection Prevention and Control Group. Outbreak of Burkholderia cepacia bacteremia in immunocompetent children caused by contaminated nebulized sulbutamol in Saudi Arabia. Am J Infect Control. 2009;37(05):431-432.
- [CrossRef] [PubMed] [Google Scholar]
- Chryseobacterium (Flavobacterium) meningosepticum outbreak associated with colonization of water taps in a neonatal intensive care unit. J Hosp Infect. 2001;47(03):188-192.
- [CrossRef] [PubMed] [Google Scholar]
- Multiresistant Elizabethkingia meningoseptica infections in tertiary care. Med J Armed Forces India. 2015;17:282-286.
- [CrossRef] [PubMed] [Google Scholar]
- Healthcare outbreaks associated with a water reservoir and infection prevention strategies. Clin Infect Dis. 2016;62(11):1423-1435.
- [CrossRef] [PubMed] [Google Scholar]
- Predictors of mortality in bloodstream infections caused by pseudomonas aeruginosa and impact of antimicrobial resistance and bacterial virulence. Antimicrob Agents Chemother. 2020;64(02):e01759-e19.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiological investigation and successful management of a Burkholderia cepacia outbreak in a neurotrauma intensive care unit. Int J Infect Dis. 2019;79:4-11.
- [CrossRef] [PubMed] [Google Scholar]






