Translate this page into:
Cardiovascular Risk Predictors High Sensitivity C-Reactive Protein and Plasminogen Activator Inhibitor-1 in Women with Lean Phenotype of Polycystic Ovarian Syndrome: A Prospective Case-Control Study
Address for correspondence: Arpita Suri, MD, DNB, MNAMS, Department of Biochemistry, Faculty of Medicine and Health Sciences, Shree Guru Gobind Singh Tricentenary (SGT) University, Gurugram 122505, Haryana, India (e-mail: arpita.lhmc@gmail.com).
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective
Accumulating evidence suggests increased cardiovascular risk in women with polycystic ovarian syndrome (PCOS) due to a cluster of factors, such as obesity, lipid abnormalities, impaired glucose tolerance (IGT), and hypertension. Markers such as high-sensitivity C-reactive protein (hs-CRP) and plasminogen activator inhibitor-1 (PAI-1) can provide an adjunctive method for the assessment of cardiovascular risk and can indicate future coronary heart diseases in women with lean PCOS.
Materials and Methods
In this prospective case-control study, women clinically diagnosed with PCOS (n = 25) with normal body mass index (BMI) and age and BMI-matched healthy controls (n = 75) were enrolled. The quantitative data were expressed as mean ± standard deviation (SD). Unpaired Student's t-test was used to compare the values (PCOS vs. controls) and Pearson's correlation coefficient was used to elucidate the relationship between the variables.
Results
The mean level of fasting blood sugar, serum total cholesterol, low-density lipoprotein (LDL), thyroid-stimulating hormone (TSH), follicle-stimulating hormone (FSH), luteinizing hormone (LH), prolactin (PRL), hs-CRP, and PAI-1 were significantly increased in PCOS patients (p < 0.000) compared with the control patients. Of the reported cases, 54.16% had hs-CRP levels greater than 3 mg/L. When the cases were further divided into normal (n = 20) and IGT (n = 5), hs-CRP values were significantly higher in IGT group as compared with normal glucose tolerance (NGT) group. On bivariate correlation analysis, hs-CRP had significant correlations with PAI-1 (r = 0.41, p < 0.000), waist-to-hip ratio (WHR; r = 0.23, p = 0.02), fasting blood sugar (FBS; r = 0.26, p = 0.009), LDL (r = 0.20, p = 0.03), TSH (r = 0.42, p < 0.000), and LH-to-FSH ratio (r = 0.24, p = 0.01).
Conclusion
Women with lean phenotype of PCOS suffer from many metabolic abnormalities such as abdominal obesity, dyslipidemia, hyperandrogenemia, and insulin resistance. The findings of the study suggest that environment of ongoing low-grade inflammation due to infiltration further exacerbates the metabolic derangements and cardiovascular risk. The investigations as hs-CRP and PAI-1 will help in early identification, diagnosis, and management of cardiovascular diseases associated with lean type of PCOS. These markers can prove to be beneficial in monitoring any unfavorable changes in cardiometabolic profile of such patients.
Keywords
cardiovascular disease
hs-CRP
lean phenotype of PCOS
PAI-1
Introduction
Polycystic ovarian syndrome (PCOS) is a heterogeneous endocrine disorder encountered in approximately 6 to 10% of women of reproductive age.[1] PCOS is a common endocrinopathy characterized by menstrual irregularity, hyperandrogenism, insulin resistance, anovulatory infertility, and multiple small ovarian cysts on ultrasonography.[2,3] There are two phenotypes of PCOS, overweight/obese and lean (normal or less BMI).[4] Further, 20 to 50% of women with PCOS have lean PCOS phenotype and the pathophysiology of this disorder may differ from their obese counterparts. A significant proportion of PCOS patients have normal body mass index (BMI; ≤ 25 kg/m2) making the diagnostic evaluation and management of such patients more difficult.[4] Therefore, the cases recruited for the study belong to lean phenotype.
PCOS is also called as Stein–Leventhal syndrome, as it was first reported in modern medical literature by Stein and Leventhal in 1935. Stein–Leventhal syndrome is the clinical manifestation of PCOS typically associated with anovulation and infertility with classical triad of oligomenorrhea or amenorrhea, hirsutism, and obesity.[5]
The aim of the present study is to evaluate the predictors of cardiovascular risk in lean PCOS. Accumulating evidence shows increased prevalence of cardiovascular disease (CVD) in women with PCOS.[6-8] Moreover, women with PCOS have a clustering of factors, such as obesity, lipid abnormalities, impaired glucose tolerance (IGT), and hypertension predisposing them to cardiovascular risk.[9] Proinflammatory markers, like high sensitivity C-reactive protein (hs-CRP), provide an adjunctive method for global assessment of cardiovascular risk[10] and determined as surrogate indicators of future coronary heart diseases in women with lean PCOS. Moreover, the relation of cardiovascular risks with lean phenotype of PCOS remains unclear in Indian population. As per our knowledge, this is the first study assessing cardiovascular risk in lean variety of PCOS. CRP is an acute-phase protein synthesized by the liver in response to factors released by adipocytes.[11] It activates the complement system and is a marker of low-grade chronic inflammation.[12] It also advances endothelial dysfunction by inducing the synthesis of soluble adhesion molecules, secreting monocyte chemoattractant protein and promoting macrophage low-density lipoprotein (LDL) uptake.[13] This study analyzes CRP in the form of hs-CRP which detects low concentrations of the protein with a higher degree of accuracy. Plasminogen activator inhibitor-1 (PAI-1) is an established risk factor for CVD.[14] Elevated plasma concentrations of PAI-1 seem to be linked to an increased risk of thrombotic vascular events and associated with an insulin resistance, abdominal obesity, metabolic syndrome,[15] and type-2 diabetes mellitus.[16] The present study was conducted to determine serum PAI-1 activity in normal-weight women with PCOS and to ascertain the role of PAI-1 as a cardiovascular risk factor in PCOS.
Materials and Methods
Study Population
All women in the age group of 18 to 35 years who attended outpatient department (OPD) in the Department of Obstetrics and Gynecology with the primary complaints of menstrual irregularities (amenorrhea or oligomenorrhea) and/or hirsutism with or without infertility were evaluated for PCOS, and women (n = 25) diagnosed with PCOS were enrolled for the study. PCOS confirmation was done by high-resolution ultrasonography in the follicular phase as per the Rotterdam revised criteria which required the presence of at least two out of three of the following features: (1) oligo- and/or anovulation, (2) clinical and/or biochemical evidence of hyperandrogenism, and/or (3) ultrasonographic findings of polycystic ovaries with exclusion of other known disorders of hyperandrogenism.[17] Selection of PCOS cases and healthy controls was done by simple random sampling. Seventy five (n = 75) healthy women were taken as BMI-matched controls as they did not have any acne, hirsutism, male-type baldness, family history of PCOS, or signs of hyperandrogenism and had regular menstrual cycles. It was a prospective case-control study performed in the Department of Biochemistry and Obstetrics and Gynecology, at Shree Guru Gobind Singh Tricentenary (SGT) Medical College, Hospital and Research Institute (SGT University), Gurgaon, Haryana, India. All PCOS women were included as per the Rotterdam revised criteria. The patients with known cases of diabetes mellitus, hypertension, severe insulin resistance, CVD, congenital adrenal hyperplasia, androgen secreting tumors, Cushing's syndrome, history of smoking and/or alcohol intake, androgenic/anabolic drug use or abuse, hyperprolactinemia, and thyroid dysfunction were excluded from the study. The size of the ovaries, volume, morphology, and the number and size of the follicles were noted. Detailed history with special reference to menstrual irregularity, hirsutism, alopecia, infertility, voice change, weight gain, presence of acne, and obstetric history was noted.
Anthropometric Parameters
Standard anthropometric data, including height, weight, waist circumference (WC), hip circumference (HC), were measured. BMI was calculated using the equation (body weight in kilograms divided by body height in meters squared; kg/m2). Asia pacific BMI guidelines by the World Health Organization (WHO) were used to identify obesity in the lean women with PCOS.[18] The waist-to-hip ratio (WHR) was measured using dressmakers tape, taking care that it was applied horizontally. Waist circumference is middle circumference between the iliac rest and the lateral costal margin and hip circumference is maximum circumference around the buttocks posteriorly and indicated anteriorly by the symphysis pubis.
Laboratory Analysis
Biochemical parameters: after 12 hours of overnight fasting, approximately 5 mL of venous blood samples were collected in plain tubes (for estimations of lipid profile and hormonal profile) and in tube containing sodium fluoride and oxalate (for plasma glucose estimation). Serum was separated and preserved at –80°C for subsequent analysis. Estimations of fasting plasma glucose, serum total cholesterol (TC), triglycerides (TG), and high-density lipoprotein (HDL) concentrations were assayed by using commercial kits available for standard photometric methods in fully automated ERBA XL (EM-200) Biochemistry analyzer. LDL was calculated by using Fredrickson–Friedewald formula.[19]
Hormonal assays: serum luteinizing hormone (LH), follicle-stimulating hormone (FSH), prolactin (PRL), and thyroid-stimulating hormone (TSH) levels were assayed by chemiluminescent immunoassay (CLIA), using Siemen's Advia Centaur CP kit. Quality controls were used to check the accuracy and precision of the analyzer, reagents, and assay results.
The study was approved by the Institutional Ethical Committee (IEC) and an informed written consent was obtained from all the participants at the study entry after apprising them the nature and objectives of the study.
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 24.0, for Windows (SPSS, Inc., Chicago, IL). The normality of distribution of all the variables was checked by using Kolmogorov–Smirnov test. The quantitative data were expressed as mean ± standard deviation (SD). Unpaired Student's t-test was used to compare the values (PCOS vs. controls), and Pearson's correlation coefficient was used to elucidate the relationship between the variables. At a confidence interval of 95%, p-values of less than 0.05 were considered statistically significant.
Results
The age of the patients ranges from 18 to 29 years with a mean age of 23 years while the age of the controls ranges from 19 to 30 years with a mean age of 23 years. Anthropometric parameters of the population calculated were height, weight, BMI, waist circumference, hip circumference and WHR as shown in ►Table 1.
| Variables | PCOS cases | Controls | p-Value |
|---|---|---|---|
| Age (y) | 22.70 ± 3.91 | 22.57 ± 2.21 | 0.83 |
| Height (m) | 1.57 ± 0.06 | 1.57 ± 0.06 | 0.73 |
| Weight (kg) | 52.39 ± 6.65 | 49.64 ± 6.43 | 0.07 |
| BMI (kg/m2) | 20.96 ± 1.62 | 20.11 ± 2.64 | 0.14 |
| Waist circumference (cm) | 30.33 ± 2.80 | 30.50 ± 2.79 | 0.80 |
| Hip circumference (cm) | 35.72 ± 2.62 | 39.10 ± 2.69 | < 0.000a |
| WHR | 0.84 ± 0.05 | 0.77 ± 0.05 | < 0.000a |
Abbreviations: BMI, body mass index; PCOS, polycystic ovarian syndrome; SD, standard deviation; WHR, waist-to-hip ratio.
a p value less than 0.001 is considered highly significant.
Biochemical and Hormonal Profile
We have evaluated both PCOS patients and controls for fasting blood sugar, lipid profile (►Fig. 1), TSH, FSH, LH, PRL, hs-CRP, and PAI-1. Comparisons were made between both the groups and the results were shown in the ►Table 2.
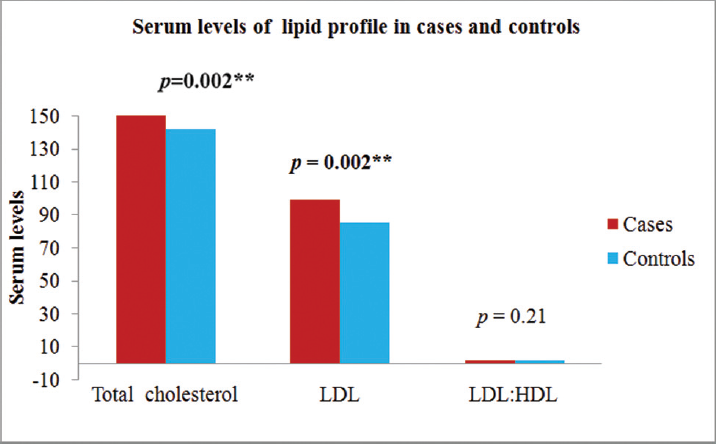
- Comparison of biochemical measurements among PCOS subjects and controls. HDL, high-density lipoprotein; LDL, low-density lipoprotein; PCOS, polycystic ovarian syndrome.
| Variables | PCOS cases | Controls | p-Value |
|---|---|---|---|
| FBS (mg/dL) | 94.16 ± 7.79 | 89.07 ± 7.45 | 0.005a |
| TC (mg/dL) | 156.41 ± 32.01 | 140.99 ± 15.44 | 0.002a |
| TG (mg/dL) | 89.50 ± 33.44 | 90.56 ± 21.82 | 0.85 |
| HDL-C (mg/dL) | 48.13 ± 10.20 | 42.91 ± 3.82 | < 0.000a |
| LDL-C (mg/dL) | 98.30 ± 31.14 | 84.50 ± 12.61 | 0.002a |
| VLDL-C (mg/dL) | 17.90 ± 6.68 | 18.11 ± 4.36 | 0.85 |
| LDL-C/HDL-C ratio | 2.13 ± 0.86 | 1.98 ± 0.33 | 0.21 |
| TC/HDL-C ratio | 3.35 ± 0.90 | 3.31 ± 0.47 | 0.78 |
| TSH (µIU/mL) | 3.37 ± 4.27 | 1.54 ± 0.83 | 0.001a |
| LH (mIU/L) | 10.73 ± 8.28 | 3.40 ± 0.70 | < 0.000a |
| FSH (mIU/L) | 5.71 ± 2.45 | 4.23 ± 0.68 | < 0.000a |
| PRL (ng/mL) | 13.80 ± 12.86 | 7.40 ± 1.17 | < 0.000a |
| LH/FSH ratio | 2.16 ± 1.54 | 0.80 ± 0.13 | < 0.000a |
| hs-CRP (mg/L) | 5.35 ± 4.24 | 2.81 ± 1.42 | < 0.000a |
| PAI-1 (ng/mL) | 9.32 ± 2.81 | 2.53 ± 1.73 | < 0.000a |
Abbreviations: FBS, fasting blood sugar; FSH, follicle-stimulating hormone; HDL-C, high-density lipoprotein- cholesterol; hs-CRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein- cholesterol; LH, luteinizing hormone; PAI-1, plasminogen activator inhibitor-1; PCOS, polycystic ovarian syndrome; PRL, prolactin; SD, standard deviation; TC, total cholesterol; TG, triglycerides; TSH, thyroid-stimulating hormone; VLDL-C, very low-density lipoprotien-cholesterol.
Note: p-Value less than 0.05 considered significant.
a p-Value less than 0.001 is considered highly significant.
The mean level of serum total cholesterol and LDL were significantly higher (p < 0.01) in cases than controls. The mean serum levels of TSH, FSH, LH, PRL, hs-CRP, and PAI-1 were significantly increased in PCOS patients (p < 0.000) compared with the control patients. The mean fasting blood sugar was significantly higher (p < 0.01) than the healthy controls. Of the total cases, 54.16% had hs-CRP levels greater than 3 mg/L which predisposes them to high cardiovascular risk.[20] The cases were further divided into normal glucose tolerance (NGT; 20 cases) and IGT (5 cases). The hs-CRP values were significantly higher in IGT group as compared with NGT group.
On bivariate correlation analysis, hs-CRP had significant positive correlations with PAI-1 (r = 0.41, p < 0.000), WHR (r = 0.23, p = 0.02), FBS (r = 0.26, p = 0.009), LDL (r = 0.20, p = 0.03), TSH (r = 0.42, p < 0.000), and LH/FSH ratio (r = 0.24, p = 0.01; ►Table 3).
| Variables | Cases | |
|---|---|---|
| r-Value | p-Value | |
| PAI-1 | 0.41 | 0.000b |
| WHR | 0.23 | 0.02a |
| FBS | 0.26 | 0.009b |
| LDL | 0.2 | 0.03a |
| TSH | 0.42 | 0.000b |
| LH/FSH ratio | 0.24 | 0.01a |
Abbreviations: FBS, fasting blood sugar; FSH, follicle-stimulating hormone; hs-CRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; LH, luteinizing hormone; PAI-1, plasminogen activator inhibitor-1; TSH, thyroid-stimulating hormone; WHR, waist-to-hip ratio.
a p-Value less than 0.05 considered significant.
b p-Value less than 0.001 is considered highly significant.
Discussion
The WHR was significantly higher in lean PCOS women as compared with age- and BMI-matched healthy controls. The WHR forms an important component of metabolic syndrome. Insulin resistance and hyperinsulinemia seen in PCOS lead to increased circulating androgens by increasing their production from theca cells.[21] The hyperandrogenemia predisposes to dyslipidemia and central adiposity. In 2012, Lim et al postulated that adipose tissue dysfunction was associated with features of metabolic syndrome which were more prevalent in PCOS than in controls.[22] This accumulation of visceral adipose tissue which is associated with increased WHR may be a key factor causing metabolic syndrome and low-grade chronic inflammation.[23] On Pearson's correlation analysis, WHR also shows significant correlation with hs-CRP (r = 0.22, p = 0.02). In 2009, a study, conducted by Oh et al,[23] in 39 lean PCOS patients found hs-CRP levels to be positively correlated with waist circumference (r = 0.46, p < 0.01). These phenotypes of PCOS women have a higher risk of developing metabolic syndrome and greater long-term risk of CVD.
The mean level of serum total cholesterol and LDL were significantly higher (p < 0.01) in cases than controls. Studies suggest that women with PCOS exhibit features of the metabolic syndrome, especially dyslipidemia which is found in majority of cases.[25-28] In 2012, Xia et al proposed that LAP was a powerful index for detection of insulin resistance in nondiabetic individuals.[29] In 2009, Wiltgen et al suggested a positive association of LAP with Homeostatic Model Assessment of Insulin Resistance (HOMA) index in PCOS and postulated that it can accurately assess insulin resistance in PCOS women.[30]
The mean serum levels of TSH was increased in lean PCOS patients (p < 0.000) compared with the control patients. Association of raised TSH with lean phenotype of PCOS can be attributed to insulin resistance as BMI is in the less or normal range. In 2009, Maratou et al suggested that increased insulin resistance can be due to defective glucose transporter (GLUT)-4 glucose transporter translocation.[31] Moreover, in this condition, there is decreased deiodinase-2 activity in pituitary due to unknown reasons.[32] This can lead to increase in TSH due to low T3 levels. In addition to this, studies suggest that females with PCOS have higher levels of thyroid antibody levels as compared with controls suggestive of autoimmune thyroiditis.[33] Moreover, PCOS in hyperestrogenic state is known to trigger autoimmunity due to proliferation of B-lymphocytes, T-cells, and macrophages as they have estrogen receptors.[34]
The mean level of hs-CRP was higher in lean PCOS women as compared with age- and BMI-matched healthy women. This significant difference can be attributed to association of chronic low-grade inflammation with PCOS.[29] This proinflammatory state causes adipose tissue dysfunction and altered adipocytokine profile which is implicated in causing insulin resistance. Moreover, lean women with PCOS have increased oxidative stress due to glucose ingestion which is known to activate nuclear factor (NF)-kB which is family of transcription factors modulating genes, such as tumor necrosis factor (TNF)-a, interleukins, and transforming growth factor β.[35] This triggers proatherogenic inflammation causing insulin resistance and hyperandrogenism independent of obesity in lean phenotype of PCOS.[36]
Our observation of increased hs-CRP in lean PCOS women as compared with age- and BMI-matched controls is in accordance to the previous studies. In 2009, Oh et al recruited 39 lean PCOS patients and 24 controls and hypothesized higher levels of hs-CRP in women with PCOS; however, the difference became insignificant after adjusting BMI.[30] In 2011, Makedos et al also conducted a study on 188 normal weight PCOS (BMI < 25 kg/m2) and postulated that hs-CRP was significantly higher in cases as compared with controls.[37] Likewise, in 2014, Keskin Kur et al also concluded that hs-CRP was significantly higher in lean PCOS women as compared with controls.[38] In addition to this, hs-CRP was significantly higher in IGT as compared with NGT women with PCOS underlying the importance of glucose metabolism in propagating chronic low-grade inflammation. Similar findings were shown by Kim et al in 2012 who also concluded that lean PCOS patients were predisposed to IGT.[39]
The hs-CRP showed a significant positive correlation with WHR, FBS, and LDL. This shows low-grade subclinical inflammation that can be linked to visceral obesity and insulin resistance as shown in previous studies. Insulin resistance leads to hyperandrogenemia which predisposes the female to abdominal obesity. The adipocytokines thus, released from adipose tissue, can initiate and maintain chronic inflammation predisposing to increased cardiometabolic risk in the lean phenotype of PCOS.[40]
The level of PAI-1 was increased in women with lean PCOS as compared with BMI-matched controls. Previous studies have suggested that plasmin plays an important role in follicular development and rupture. In 1996, Sampson et al also suggested that high PAI-1 levels can delay maturation and hence can develop ovarian maturation.[41] Insulin is known to increase hepatic PAI-1 production. Therefore, PCOS, a state of hyperinsulinemia may have the involvement of PAI-1 as a primary event in anovulation. Similar findings were reported by Elci et al in 2016 who stated that cardiovascular risk marker PAI-1 was significantly elevated in nonobese women (BMI < 30 kg/m2) with PCOS.[42] In 2004, Orio et al also suggested significantly higher levels of PAI-1 in normal-weight PCOS women as compared with controls.[43]
Strengths and Limitations
Our study focused on lean phenotype of PCOS on which limited studies have been done in India. Additionally, we further segregated our cases on the basis of normal and IGT to study the effect of glucose tolerance on the hs-CRP.
However, there were few limitations in our study. Sample size in our study was limited to 25 nonobese women with PCOS as BMI 23 kg/m2 or lower was our inclusion criteria for cases. In addition to this, we could not calculate insulin resistance to correlate with hs-CRP and PAI-1 in our study. Thus, studies with larger sample size would further elucidate and validate the relation of cardiovascular risk with lean phenotype of PCOS.
Conclusion
Women with lean phenotype of PCOS suffer from many metabolic abnormalities like abdominal obesity, dyslipidemia, hyperandrogenemia, and insulin resistance. The findings of the study suggest that environment of ongoing low-grade inflammation due to infiltration of macrophages in peripheral tissues further exacerbates the metabolic derangements and cardiovascular risk. The investigations as hs-CRP and PAI-1 will help in early identification, diagnosis, and management of CVDs associated with lean type of PCOS. These markers can prove to be beneficial in monitoring any unfavorable changes in cardiometabolic profile of such patients
Conflict of Interest
None declared.
Funding
None.
References
- The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89(06):2745-2749.
- [CrossRef] [PubMed] [Google Scholar]
- Polycystic ovary syndrome. N Engl J Med. 2005;352(12):1223-1236.
- [CrossRef] [PubMed] [Google Scholar]
- Lean polycystic ovary syndrome (PCOS): an evidence-based practical approach. J Diabetes Metab Disord. 2018;17(02):277-285.
- [CrossRef] [PubMed] [Google Scholar]
- Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29(02):181-191.
- [CrossRef] [Google Scholar]
- Adverse lipid and coronary heart disease risk profiles in young women with polycystic ovary syndrome: results of a case-control study. J Clin Epidemiol. 1998;51(05):415-422.
- [CrossRef] [PubMed] [Google Scholar]
- Evidence for association between polycystic ovary syndrome and premature carotid atherosclerosis in middle-aged women. Arterioscler Thromb Vasc Biol. 2000;20(11):2414-2421.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiovascular disease in women with polycystic ovary syndrome at long-term follow-up: a retrospective cohort study. Clin Endocrinol (Oxf). 2000;52(05):595-600.
- [CrossRef] [PubMed] [Google Scholar]
- Polycystic ovary syndrome: a major unrecognized cardiovascular risk factor in women. Rev Obstet Gynecol. 2009;2(04):232-239.
- [Google Scholar]
- C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836-843.
- [CrossRef] [PubMed] [Google Scholar]
- Mediators of chronic inflammation in polycystic ovarian syndrome. Gynecol Endocrinol. 2012;28(12):974-978.
- [CrossRef] [PubMed] [Google Scholar]
- Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754.
- [CrossRef] [PubMed] [Google Scholar]
- Modulation of C-reactive protein-mediated monocyte chemoattractant protein-1 induction in human endothelial cells by anti-atherosclerosis drugs. Circulation. 2001;103(21):2531-2534.
- [CrossRef] [PubMed] [Google Scholar]
- Plasminogen-activator inhibitor type 1 and coronary artery disease. N Engl J Med. 2000;342(24):1792-1801.
- [CrossRef] [PubMed] [Google Scholar]
- PAI-1 and the metabolic syndrome: links, causes, and consequences. Arterioscler Thromb Vasc Biol. 2006;26(10):2200-2207.
- [CrossRef] [PubMed] [Google Scholar]
- PAI-1 and diabetes: a journey from the bench to the bedside. Diabetes Care. 2012;35(10):1961-1967.
- [CrossRef] [PubMed] [Google Scholar]
- Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(01):19-25.
- [CrossRef] [Google Scholar]
- The Asia-Pacific perspective: redefining obesity and its treatment. Available at: 0957708211_eng.pdf (who.int). Accessed March 24, 2022
- [Google Scholar]
- Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(06):499-502.
- [Google Scholar]
- Increased C-reactive protein levels in the polycystic ovary syndrome: a marker of cardiovascular disease. J Clin Endocrinol Metab. 2004;89(05):2160-2165.
- [CrossRef] [PubMed] [Google Scholar]
- Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2012;18(06):618-637.
- [CrossRef] [PubMed] [Google Scholar]
- Low grade chronic inflammation in women with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2001;86(06):2453-2455.
- [CrossRef] [PubMed] [Google Scholar]
- Serum C-reactive protein levels in normal-weight polycystic ovary syndrome. Korean J Intern Med (Korean Assoc Intern Med). 2009;24(04):350-355.
- [CrossRef] [PubMed] [Google Scholar]
- PCOS/Troglitazone Study Group. Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91(01):48-53.
- [CrossRef] [PubMed] [Google Scholar]
- The prevalence of polycystic ovary syndrome in reproductive-aged women of different ethnicity: a systematic review and meta-analysis. Oncotarget. 2017;8(56):96351-96358.
- [CrossRef] [PubMed] [Google Scholar]
- The prevalence of metabolic disorders in various phenotypes of polycystic ovary syndrome: a community based study in Southwest of Iran. Reprod Biol Endocrinol. 2014;12(01):89.
- [CrossRef] [PubMed] [Google Scholar]
- Association of hormonal status with anthropometric & biochemical parameters in women with polycystic ovarian syndrome. J Comm Health Management. 2017;4(01):30-34.
- [Google Scholar]
- Study of anthropometric measurements, biochemical parameters and hormonal levels in women with PCOS at a tertiary center of rural Haryana. J Evol Med Dent Sci. 2019;8(16):1311-1317.
- [CrossRef] [Google Scholar]
- Lipid accumulation product is a powerful index for recognizing insulin resistance in non-diabetic individuals. Eur J Clin Nutr. 2012;66(09):1035-1038.
- [CrossRef] [PubMed] [Google Scholar]
- Lipid accumulation product index: a reliable marker of cardiovascular risk in polycystic ovary syndrome. Hum Reprod. 2009;24(07):1726-1731.
- [CrossRef] [PubMed] [Google Scholar]
- Studies of insulin resistance in patients with clinical and subclinical hypothyroidism. Eur J Endocrinol. 2009;160(05):785-790.
- [CrossRef] [PubMed] [Google Scholar]
- High-normal TSH values in obesity: is it insulin resistance or adipose tissue's guilt? Obesity (Silver Spring). 2013;21(01):101-106.
- [CrossRef] [PubMed] [Google Scholar]
- High prevalence of autoimmune thyroiditis in patients with polycystic ovary syndrome. Eur J Endocrinol. 2004;150(03):363-369.
- [CrossRef] [PubMed] [Google Scholar]
- Women and autoimmune diseases. Emerg Infect Dis. 2004;10(11):2005-2011.
- [CrossRef] [PubMed] [Google Scholar]
- Increased activation of nuclear factor kappaB triggers inflammation and insulin resistance in polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91(04):1508-1512.
- [CrossRef] [PubMed] [Google Scholar]
- Inflammation in polycystic ovary syndrome: underpinning of insulin resistance and ovarian dysfunction. Steroids. 2012;77(04):300-305.
- [CrossRef] [PubMed] [Google Scholar]
- Increased serum C-reactive protein levels in normal weight women with polycystic ovary syndrome. Hippokratia. 2011;15(04):323-326.
- [Google Scholar]
- The effect of obesity on inflammatory markers in patients with PCOS: a BMI-matched case-control study. Arch Gynecol Obstet. 2014;290(02):315-319.
- [CrossRef] [PubMed] [Google Scholar]
- High sensitivity C-reactive protein and its relationship with impaired glucose regulation in lean patients with polycystic ovary syndrome. Gynecol Endocrinol. 2012;28(04):259-263.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of clinical, biochemical, and hormonal profile of lean versus overweight polycystic ovarian syndrome patients: a cross-sectional study. J Intern Med. 2022;10(01):13-16.
- [CrossRef] [Google Scholar]
- Ambulatory blood pressure profiles and plasminogen activator inhibitor (PAI-1) activity in lean women with and without the polycystic ovary syndrome. Clin Endocrinol (Oxf). 1996;45(05):623-629.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of cardiac risk marker levels in obese and non-obese patients with polycystic ovaries. Gynecol Endocrinol. 2017;33(01):43-47.
- [CrossRef] [PubMed] [Google Scholar]
- Is plasminogen activator inhibitor-1 a cardiovascular risk factor in young women with polycystic ovary syndrome? Reprod Biomed Online. 2004;9(05):505-510.
- [CrossRef] [PubMed] [Google Scholar]





