Translate this page into:
Assessment of in vitro antimicrobial activity of ceftazidime-avibactam and phenotypic synergy testing with aztreonam against carbapenem resistant Gram-negative bacilli in a tertiary care hospital
*Corresponding author: Sarita Otta, Department of Microbiology, Institute of Medical Sciences and SUM Hospital, Bhubaneswar, Odisha, India. saritaotta@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Mishra S, Bhoi P, Choudhary L, Panigrahi R, Otta S. Assessment of in vitro antimicrobial activity of ceftazidimeavibactam and phenotypic synergy testing with aztreonam against carbapenem resistant Gram-negative bacilli in a tertiary care hospital. J Lab Physicians. 2024;16:366-71. doi: 10.25259/JLP_30_2024
Abstract
Objectives:
Ceftazidime avibactam (CZA) is a drug used against carbapenemase producing Gram-negative bacterial infections. Avibactam (AVI) is a non-beta-lactam-beta-lactamase inhibitor which has no action against metallo-β-lactamase (MBL) enzymes. This inadequacy is counteracted by combining CZA with aztreonam (ATM). The present study aims to denote the in vitro susceptibility pattern of the CZA and CZA-ATM combination in our area.
Materials and Methods:
In this study conducted prospectively from January to June 2023, the samples growing Enterobacterales and Pseudomonas aeruginosa were proceeded for carbapenemase detection by phenotypic testing for EDTA carbapenem inactivation method and modified carbapenem inactivation method. The minimum inhibitory concentration MIC of CZA was determined by E-strip and interpreted as per clinical and laboratory standard institute (CLSI) guidelines, while synergy testing of CZA and ATM was performed using ATM discs.
Statistical Analysis:
All data were entered in Microsoft Excel and analyzed for basic statistics.
Results:
The study included 150 carbapenem resistant organisms (131 Enterobactarales and 19 P. aeruginosa). Among these Enterobacterale strains, 72 (54.9%) were MBL producers. CZA resistance was detected in 69.3% of Klebsiella spp., 61.53% of Escherichia coli, and 50% of Serratia spp. Among Klebsiella spp. and E. coli, 88.9% and 65.2% of MBL isolates showed in vitro synergy to CZA-ATM.
Conclusions:
The study highlights a good in vitro sensitivity pattern of the CZA and ATM combination. However, we also highlight a growing percentage of non-synergistic interactions that need further genetic evaluation.
Keywords
Ceftazidime avibactam
Aztreonam
Metallo-beta-lactamase
Modified carbapenem inactivation method
INTRODUCTION
Carbapenem resistance is on the rise among the Enterobactarales (CRE) and Pseudomonas spp. carbapenem-resistant Pseudomonas aeruginosa (CRPA). These isolates are often resistant to many other classes of antimicrobials, thus lessening the therapeutic options.[1] Ceftazidime avibactam (CZA) is one of the molecules used against such carbapenemase producing Gram-negative bacterial infections and is often heralded for its in vitro sensitivity and better clinical outcome.[2] Avibactam (AVI) is a non-beta-lactam-beta-lactamase inhibitor having activity against serine carbapenemases like Klebsiella pneumoniae carbapenemase (KPC) and OXA (oxacillinase)-48-like carbapenemases while it has no action against metallo-β-lactamase (MBL) enzymes. However, this selective activity has created a potential void in areas like India where the MBL carbapenemases account for a vast proportion of about 61% of CRE and 15–30% of CRPA.[3]
The age-old drug aztreonam is stable against MBLs due to its potent affinity for penicillin binding protein-3 (PBP3), but its therapeutic benefit is impeded by the fact that MBL producers often secrete other class A, C, or D enzymes against whom it is ineffective.[4,5] Thus, a clever combination of CZA and aztreonam has been designed, where AVI neutralizes class A (Extended Spectrum β Lactamases [ESBL] and KPC), C enzymes, and D (OXA-48-like) enzymes and aztreonam the MBLs. The utility of this drug combination is limited in MBL-producing P. aeruginosa and Acinetobacter spp., as aztreonam may be ejected out of the cell by the efflux pump in these organisms. This drug combination has provided a glimmer of hope in the antibiotic horizon. The Infectious Diseases Society of America recommends that CZA and aztreonam infusion can be used concurrently for MBL organisms.[6] However, recent studies have also started reporting decreased susceptibility to ATM-CZA that has been noted in studies due to a small insertion into in PBP3 that impacts the binding of ATM, ceftazidime, and other β-lactams.[7] Thus, it is important to note the susceptibility pattern of this synergistic combination in clinical microbiology laboratories.
There is no clinical and laboratory standard institute (CLSI) approved method for evaluation of synergy testing of the said drugs. However, studies have shown many effective methods, for example, determining aztreonam minimum inhibitory concentration (MIC) by broth microdilution (BMD) when the broth has 4 mg/L of AVI.[8] MIC determination using E-strips by CZA and ATM strip stacking and strip crossing methods[9] overlay of aztreonam (30 μg) disc and CZA disk (30/20 μg) disc.[9] A disk elution method has been proposed, which has a good correlation with the BMD method.
In view of the lack of published literature describing the in vitro susceptibility pattern of CZA and CZA-ATM combination in our area, the present study was undertaken to denote the in vitro susceptibility pattern of CZA and CZAATM combination.
MATERIALS AND METHODS
Study setting
The present study was undertaken prospectively in a premier tertiary care teaching hospital in Odisha, in the eastern part of India, from January to June 2023. All the samples were received in a central laboratory, where the organisms were isolated and identified. In our setup, an Automated Vitek 2 bioMerieux system was used for performing the identification and sensitivity of the different organisms.
Inclusion criteria
All the non-repetitive clinically significant carbapenemase producing isolates of Enterobactarales, P. aeruginosa from various intensive care units (ICU) of our hospital during the study period were included in the study.
Phenotypic detection of MBLs in Enterobactarales
Carbapenem resistance was detected when MIC for either imipenem or meropenem was ≥4 μg/mL.[10] The carbapenem inactivation method (CIM), as recommended by CLSI., was adopted for phenotypic detection of carbapenemases. Modified CIM (mCIM) method was used for carbapenemases detection in organisms belonging to Enterobacterales following which EDTA CIM (eCIM) was used to differentiate MBL from serine protease. The test is based on the principle that ethylenediaminetetraacetic acid (EDTA), being a metal chelator, inactivates the zinc molecule present in MBL, thereby inactivating it.
Fresh colonies of the test organism (Enterobacterales 1 μL loopful) were taken from an overnight incubated blood agar plate and emulsified in two test tubes (one each for eCIM and m CIM) of 2 mL Trypticase Soy broth for each isolate. For e CIM, 20 μL of 0.5M EDTA was prepared by dissolving 18.6 g of EDTA.Na2.2H2O in 100 mL of distilled water was added before addition of the test organism. Both the broths were vortexed for 15 seconds, and then, a 10μg meropenem disc was added to each of the two tubes. This was incubated at 37°C for 4 h in ambient air, following which the meropenem discs were taken out with the help of a sterile loop, and excess inoculums were decanted off by pressing the disc against the sides of the test tube. These disks were placed on a lawn culture of American type culture collection (ATCC) Escherichia coli 25,922.
If the zone around the meropenem of the mCIM test was resistant, the isolate was interpreted as positive for the production of carbapenemase. eCIM test was interpreted only when the mCIM was positive. A ≥5 mm increase in eCIM zone in comparison to mCIM was considered positive for MBL. A mCIM positive with eCIM negative isolate was taken as having a carbapenemase other than MBL.
Phenotypic detection of susceptibility of CZA and colistin
CZA E strips obtained from Himedia were used to determine this combination’s MIC. The CLSI breakpoint of 8 μg/mL was used for interpretation. Colistin susceptibility testing was performed using the colistin broth disc elution method.
Phenotypic detection of synergy between CZA and aztreonam
MBL positive Enterobacterale isolates and all carbapenem resistant Pseudomonas isolates were further tested for synergy between CZA and ATM using CZA E-strip (HiMedia Labs) and 30 μg ATM disks (HiMedia Labs). On a mueller hinton agar (MHA) plate inoculated with an MBL positive isolate, a CZA E-strip is placed, and another ATM disc is placed 15 mm away from at center, taking into consideration that center of the disc remains parallel to the cutoff sensitivity mark of CZA (8 μg/mL). After overnight incubation, the results were interpreted as per CLSI breakpoints for CZA and ATM. Augmentation of the ATM zone towards the CZA strip was considered positive for synergy between these two antibiotics [Figure 1].
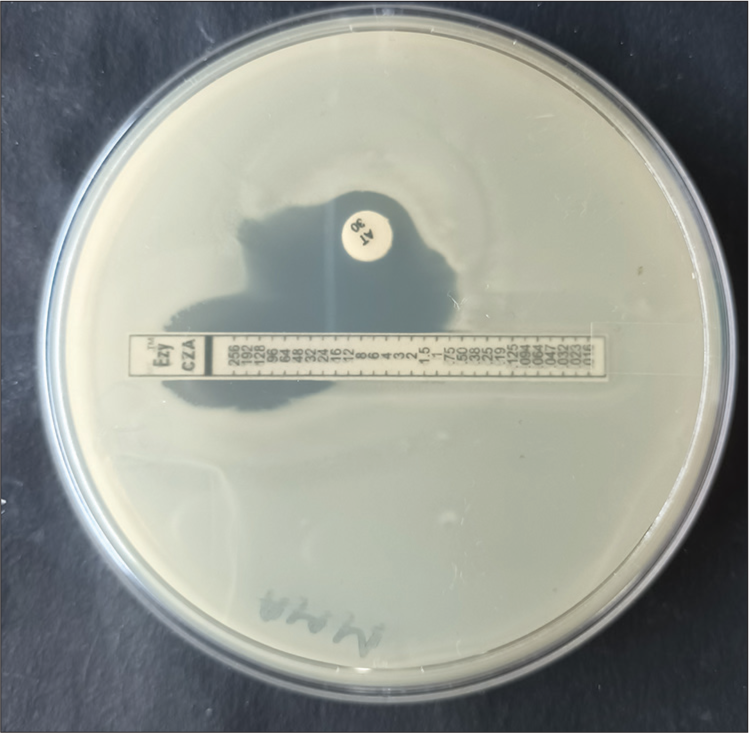
- Picture showing augmentation of aztreonam zone toward ceftazidime avibactam E-strip, thus positive for synergy between these two antibiotics.
All the data obtained were entered into an Excel sheet, and data were interpreted by basic statistical parameters.
RESULTS
During the study period, 150 carbapenem resistant organisms, including Enterobactarales (131, 87.3%) and P. aeruginosa (19, 12.7 %), were isolated from various samples. Enterobactarales isolated included-K. pneumoniae (101, 67.3%), E. coli (26, 17.3%), and Serratia spp. (4, 2.7%). These organisms were commonly isolated from blood 61 (46.6 %), followed by tracheal aspirates 32 (24.4%) [Table 1].
| Organism | No (% total) |
Blood (% total) |
Tracheal aspirate (% total) | Pus (% total) |
Sputum (% total) |
Urine (% total) |
Tissue (% total) |
Oral swab (% total) |
Bile (% total) |
Ascitic fluid (% total) |
|---|---|---|---|---|---|---|---|---|---|---|
| Klebsiella spp. | 101 (67.3) | 49.5 | 33.66 | 4.95 | 6.93 | - | 1 | 2.97 | - | 1 |
| Escherichia coli | 26 (17.3) | 30.77 | 23.08 | 19.23 | - | 3.84 | 11.54 | - | 3.85 | 7.69 |
| Serratia marcescens | 4 (2.7) | 75 | 25 | - | - | - | - | - | - | - |
| Pseudomonas aeruginosa | 19 (12.7) | 10.5 | 36.84 | 15.79 | 21.05 | 5.26 | 10.52 | - | - | - |
| Total | 150 | 61 (46.6) | 32 (24.4) | 20 (13.3) | 7 (4.7) | 4 (2.7) | 4 (2.7) | 2 (1.3) | 1 (0.7) | 3 (0.02) |
Colistin and polymyxin B were the most sensitive antibiotics for all the carbapenem resistant organisms. Klebsiella spp. showed dismal susceptibility to all the antibiotics, including cefepime, aminoglycosides, chloramphenicol, and tetracycline. E. coli isolates were sensitive to tetracycline (57.7%), doxycycline (53.8%), and netilmicin (65.4%). Pseudomonas spp. was susceptible to netilmicin and tobramycin (42.1%) [Table 2].
| CZA | C | CL* | CLT | CFX | DO | CFP | LE | NET | PB | AMS | TE | TOB | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Klebsiella spp. (n=101) | 30.7 | 17.8 | 89.1 | 10.9 | 12.9 | 31.7 | 3.9 | 6.9 | 15.8 | 89.1 | 1.9 | 25.7 | 11.9 |
| Escherichia coli (n=26) | 38.5 | 19.2 | 96.2 | 26.9 | 30.8 | 53.8 | 19.2 | 11.5 | 65.4 | 100 | 23.1 | 57.7 | 46.2 |
| Serratia spp. (n=4) | 50 | 75 | IR | 50 | 75 | 75 | 25 | 50 | 50 | IR | IR | 50 | 50 |
| Pseudomonas aeruginosa (n=19) | 36.8 | - | 78.9 | 36.8 | - | - | 36.8 | 31.6 | 42.1 | 89.5 | - | - | 42.1 |
C: Chloramphenicol, CL: Colistin, CLT: Ceftolozane-tazobactam, CFX: Ceftizoxime, DO: Doxycycline, CFP: Cefepime, LE: Levofloxacin, NET: Netilmicin, PB: Polymyxin B, AMS: Ampicillin-sulbactam, TE: Tetracycline, TOB: Tobramycin, IR: Intrinsic resistance. *Colistin percentage is calculated from intermediate isolates as there is no susceptible breakpoint for this antibiotic, CZA: Ceftazidime avibactam
Among beta-lactam beta-lactamase combinations, ceftolozane tazobactam (as tested by Vitek-2) was less susceptible antibiotic to the organisms than CZA. Among Enterobactarales, 69.3% of Klebsiella spp., 61.53% of E. coli, and 50% of Serratia spp. were resistant to CZA. MIC50/MIC90 of both Klebsiella spp. and E. coli was 16/16, while that of Serratia spp. was 12/16.
On phenotypic testing of the Enterobacterale strains, 72 (54.9%) produced MBL type of carbapenemase. All the Serratia spp. and 88.5% of E. coli were MBL producers. Coproduction of ESBL enzyme was noticed in only 12.2% of tested isolates. There was a striking higher (82.4%) coproduction of Amp C type of beta-lactamases along with the carbapenemase production. Klebsiella spp. was highest producers of Amp C (85.14%) [Table 3].
| Serine carbapenemase No (%) | MBL No (%) | ESBL No (%) | Amp C No (%) | Synergy positive* (% of MBL isolates) |
|
|---|---|---|---|---|---|
| Klebsiella spp. (n=101) | 56 (55.44%) | 45 (44.55%) | 13 (12.87%) | 86 (85.14%) | 40 (88.88%) |
| Escherichia coli (n=26) | 3 (11.54%) | 23 (88.46%) | 3 (11.53%) | 19 (73.07%) | 15 (65.21%) |
| Serratia spp. (n=4) | 0 | 4 (100%) | 0 | 3 (75%) | 4 (100%) |
| Total | 59 (45%) | 72 (54.9%) | 16 (12.2%) | 108 (82.4%) | 59 |
Among the isolated organisms, 78 (77.28%) of Klebsiella spp., 12 (46.15%) of E. coli, and 9 (47.7%) of Pseudomonas spp. were multidrug-resistant (MDR) organisms. These MDR organisms also showed a high degree of resistance to CZA. Apart from 17 (21.8%) of Klebsiella spp., the rest were resistant to CZA.
Synergistic activity of CZA with ATM was determined by phenotypic method in MBL positive Enterobactarales and P. aeruginosa isolates. All the Serratia isolates showed in vitro synergistic activity of these drugs. Among Klebsiella and E. coli, 88.9% and 65.2% of MBL isolates showed in vitro synergy [Table 3].
DISCUSSION
Resistance to carbapenems is common worldwide and often mediated due to one of these mechanisms-porin mutation, overexpression of the efflux pump, and carbapenemase production. Carbapenemase production is the predominant among them and is often mediated by plasmid encoded serine enzymes such as KPC and OXA 48 of Ambler class A and D, respectively. MBLs, Ambler class B including New Delhi metallo beta- lactamase (NDM), verone integron-encoded metallo-beta-lactamase (VIM), and imipenemase (IMP) are also implicated. India has a high preponderance of NDM producing Enterobacterales. Tigecycline, polymyxin, and CZA are limited antimicrobial options available at our end for tackling CRE strains. The antibiotics such as tigecycline and colistin are associated with the emergence of drug resistance during treatment and are toxic as well and contribute to high mortality and morbidity. Tigecycline also lacks in vitro synergy, limiting its use in many situations.[11]
CZA was launched in September 2015, and it contained a novel diazabicyclooctane β-lactamase inhibitor AVI which had promising activity against Enterobacterales producing serine enzymes, that is, ESBL enzymes, KPC and OXA 48 as well as against the class C cephalosporinases of P. aeruginosa. CZA is being used for these organisms in adults with limited treatment options as seen in various hospital acquired infections.[12,13] CZA in many studies has been shown to fare superiorly than colistin the last resort drug till date in terms of amelioration of clinical symptoms and low mortality in CRE infection.[14] In vitro studies have found CZA as having the highest percentage of susceptibility against clinical isolates of Enterobacterales.[15] However, in the present study among the carbapenem resistant GNBs, 69.3% of Klebsiella spp., 61.53% of E. coli and 50% of Serratia spp. and 62.5% of P. aeruginosa were resistant to CZA. Similarly in another study,[16] the resistance to CZA was shown in 90% of CRE. This is higher in comparison to a recent study[17] where the CZA susceptibility for CRE and CRPA cases was 34% and 32%, respectively. The resistance to CZA closely correlated with MBL positivity (54.9%) in CRE cases. Although serine carbapenemase are predominant in other parts of the world, MBLs are common in Southeast Asia including India thus limiting the usefulness of this novel antibiotic.[18,19] In contrast to studies from African subcontinent,[17] the susceptibility of our isolates to carbapenem resistant P. aeruginosa is low (36.8%). This is probably due to presence of many MBL genes but there may be many other unexplored factors. We could not perform the genotypic assay to detect the MBL genes which are a limitation of the present study.
A solution to the CZA resistance was to cleverly combine CZA and ATM, which can prove as a cost-effective as well as clinically effective solution.[20] Marshall et al. had first shown in 2017 that among 21 MBL positive CZA resistant isolates, 17 responded to combination of ATM and CZA.[21] Karlowsky et al. had also demonstrated that 99.9% of isolates collected from 40 different countries were inhibited by the said combination.[22] In the present study, synergy was noted in 89% Klebsiella isolates while it was markedly less in E. coli strains (65.2%). This is much less than that noted by Taha et al.[16] where CZA and aztreonam combination showed a synergistic effect in 98.8% of Klebsiella spp. and 95% of E coli. Sreenivasan et al.[23] also showed 100% in vitro synergy of this combination in MBL positive Enterobactarales. Few recent studies had also demonstrated the synergy between CAZ/AVI and ATM in MDR, extentensive drug resistant (XDR) and pan drug resistant (PDR) isolates using different phenotypic techniques.[8,9,16,21,23,24] The previous studies[7,25] have also reported the resistance to this combination due to change in PBP3 protein.
Our study has several limitations like-not performing the genotypic evaluation of the carbapenem resistant isolates. Inclusion of samples from a single tertiary care set up, no data regarding the clinical effectiveness of this drug combination could be collected. We also did not perform the checkerboard method the current method of choice for in vitro testing of combination regimens.
CONCLUSIONS
This is one of the foremost studies depicting the resistance pattern of CZA, a commonly used drug in ICU setting and highlights the present state of synergistic combination with aztreonam in our area. MBL positive Klebsiella and E. coli show 88.9% and 65.2% in vitro sensitivity, respectively, to this combination which is lesser than many previous studies. Further, evaluation to denote the genetic basis of this non-synergistic interaction should be looked into in.
Ethical approval
The study was approved by the Institutional Ethical Board of the hospital via no. IEC/IMS.SH/SOA/2023/350.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis. 2013;13:785-96.
- [CrossRef] [PubMed] [Google Scholar]
- Colistin versus ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. Clin Infect Dis. 2018;66:163-71.
- [CrossRef] [PubMed] [Google Scholar]
- Establishing antimicrobial resistance surveillance and research network in India: Journey so far. Indian J Med Res. 2019;149:164-79.
- [CrossRef] [PubMed] [Google Scholar]
- Carbapenemases: The versatile beta-lactamases. Clin Microbiol Rev. 2007;20:440-58. table of contents
- [CrossRef] [PubMed] [Google Scholar]
- Infectious Diseases Society of America guidance on the treatment of extended-spectrum β-lactamase producing enterobacterales (ESBL-E), carbapenem-resistant enterobacterales (CRE), and Pseudomonas aeruginosa with difficult-to-treat resistance (DTR-P. aeruginosa) Clin Infect Dis. 2021;72:e169-83.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic features leading to reduced susceptibility to aztreonam-avibactam among metallo-βlactamase-producing Escherichia coli isolates. Antimicrob Agents Chemother. 2020;64 doi: 10.1128/AAC.01659-2
- [CrossRef] [PubMed] [Google Scholar]
- Will ceftazidime/avibactam plus aztreonam be effective for NDM and OXA-48-Like producing organisms: Lessons learnt from in vitro study. Indian J Med Microbiol. 2019;37:34-41.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of susceptibility testing methods for aztreonam and ceftazidime-avibactam combination therapy on extensively drug-resistant gram-negative organisms. Antimicrob Agents Chemother. 2021;65:e0084621.
- [CrossRef] [PubMed] [Google Scholar]
- Performance standards for antimicrobial susceptibility testing In: CLSI supplement M100 (30th ed). Wayne, Pennysalvania, USA: CLSI; 2020.
- [Google Scholar]
- Mono vs. combo regimens with novel beta-lactam/beta-lactamase inhibitor combinations for the treatment of infections due to carbapenemase-producing Enterobacterales: Insights from the literature. Infection. 2021;49:411-21.
- [CrossRef] [Google Scholar]
- AVYCAZ® package insert. 2022. Available from: https://www.allergan.com/assets/pdf/avycaz-pi [Last accessed on 2024 Feb 29]
- [Google Scholar]
- Zavicefta summary of product characteristics. Available from: https://www.ema.europa.eu/en/documents/product-information/zavicefta-epar-product-information_en.pdf [Last accessed on 2024 Feb 29]
- [Google Scholar]
- Effectiveness of ceftazidime-avibactam versus colistin in treating carbapenem-resistant Enterobacteriaceae bacteremia. Int J Infect Dis. 2021;109:1-7.
- [CrossRef] [PubMed] [Google Scholar]
- In vitro susceptibility to ceftazidime/avibactam and comparators in clinical isolates of enterobacterales from five Latin American countries. Antibiotics (Basel). 2020;9:62.
- [CrossRef] [PubMed] [Google Scholar]
- Ceftazidime-Avibactam plus aztreonam synergistic combination tested against carbapenem-resistant Enterobacterales characterized phenotypically and genotypically: A glimmer of hope. Ann Clin Microbiol Antimicrob. 2023;22:21.
- [CrossRef] [PubMed] [Google Scholar]
- In vitro activity of ceftazidime-avibactam against clinical isolates of Enterobacterales and Pseudomonas aeruginosa from sub-Saharan Africa: ATLAS global surveillance program 2017-2021. J Glob Antimicrob Resist. 2023;35:93-100.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular characterization of carbapenem-resistant Enterobacteriaceae at a tertiary care laboratory in Mumbai. Eur J Clin Microbiol Infect Dis. 2015;34:467-72.
- [CrossRef] [PubMed] [Google Scholar]
- The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence. 2017;8:460-9.
- [CrossRef] [PubMed] [Google Scholar]
- Fosfomycin resistance in Escherichia coli isolates from South Korea and in vitro activity of fosfomycin alone and in combination with other antibiotics. Antibiotics (Basel). 2020;9:112.
- [CrossRef] [PubMed] [Google Scholar]
- Can ceftazidime-avibactam and aztreonam overcome β-lactam resistance conferred by metallo-β-lactamases in Enterobacteriaceae? Antimicrob Agents Chemother. 2017;61:e02243-16.
- [CrossRef] [PubMed] [Google Scholar]
- In vitro activity of aztreonam-avibactam against Enterobacteriaceae and Pseudomonas aeruginosa isolated by clinical laboratories in 40 countries from 2012 to 2015. Antimicrob Agents Chemother. 2017;61:472-17.
- [CrossRef] [PubMed] [Google Scholar]
- In-vitro susceptibility testing methods for the combination of ceftazidime-avibactam with aztreonam in metallobetalactamase producing organisms: Role of combination drugs in antibiotic resistance era. J Antibiot (Tokyo). 2022;75:454-62.
- [CrossRef] [PubMed] [Google Scholar]
- Synergistic activity of ceftazidime-avibactam and aztreonam against serine and metallo-β-lactamase-producing gram-negative pathogens. Diagn Microbiol Infect Dis. 2017;88:352-4.
- [CrossRef] [PubMed] [Google Scholar]
- In vitro selection of aztreonam/avibactam resistance in dual-carbapenemase-producing Klebsiella pneumoniae. J Antimicrob Chemother. 2020;75:559-65.
- [CrossRef] [PubMed] [Google Scholar]






