Translate this page into:
Clinico-microbiological profile of urosepsis patients in a tertiary care hospital in India: A 1-year study
*Corresponding author: Kavitha Prabhu, Assistant Professor, Father Muller Medical College, Mangaluru, Karnataka, India kaavitaramesh@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: Prabhu K, Bhat PN, Boloor R, Shetty AK, Nayak G, Sundarsingh V. Clinico-microbiological profile of urosepsis patients in a tertiary care hospital in India: A 1-year study. J Lab Physicians. 2024;16:387-92. doi: 10.25259/JLP_25_2024
Abstract
Objectives:
Urosepsis is a life-threatening bacterial infection resulting from a complicated urinary tract infection. Early diagnosis of urosepsis, its causative agent, and the susceptibility pattern are most important for accurate treatment to prevent mortality. Diagnosis includes recognition of the presence of sepsis and the investigations that help in the management of patients. The most common organism causing urosepsis is Escherichia coli, followed by other members of the Enterobacteriaceae family. With this background, this study was conducted to determine the clinical-microbiological profile of urosepsis patients in our tertiary care hospital.
Materials and Methods:
This was a prospective observational study; patients with clinical urosepsis and growing identical bacteria from both urine and blood cultures taken simultaneously are included in the study. The details of the clinical presentation and laboratory investigation results were recorded on an Excel sheet.
Statistical analysis:
Statistical analysis was done using IBM Statistical Package for the Social Sciences Statistics V.23. The categorical variables such as demographic, microbiological, and other laboratory characteristics and clinical outcomes were analyzed and expressed in terms of frequencies and percentages. Continuous variables were expressed in terms of median. Independent t-test, Mann–Whitney U-test, Chi-square test, or Fisher’s exact test were used wherever applicable.
Results:
We found 87 urosepsis patients in one year with a mortality rate of 22.98%. The mean age of the patients was 61.5 years, with majority (42.42%) above 65 years. Gram-negative bacilli were frequently isolated, with the highest number of E. coli (68.96%), followed by Klebsiella pneumoniae (20.68%). Sixteen (18.4%) of Gram-negative bacilli were Carbapenem-resistant Enterobacteriaceae. Multiple risk factors were seen in 58/87 (66.66%) patients, with diabetes mellitus as the most common risk factor.
Conclusions:
Urosepsis is a critical condition with a high mortality rate. Meropenem can be used as an empirical therapy with careful observation of patients in view of the occurrence of carbapenem resistance. A multidisciplinary team approach comprising intensive care specialists, urologists, radiologists, and microbiologists is very important for the effective and rapid management of urosepsis.
Keywords
Urosepsis
Gram-negative bacilli
antimicrobial resistance
INTRODUCTION
Urosepsis is sepsis which is caused by the complication of the urogenital tract infection leading to a systemic response. When there is associated organ dysfunction, it is referred to as severe sepsis, and when there is hypotension and hypoperfusion requiring vasopressor therapy, then it is regarded as septic shock.[1,2] The incidence of urosepsis increases with risk factors such as diabetes mellitus, age (≥65 years), female patients, immune suppression (organ transplantation, chemotherapy, corticosteroid treatment, acquired immune deficiency syndrome), hospital-acquired urinary tract infection (UTI), and prior urological interventions.[3-5] Urosepsis must be detected at an early stage and promptly treated with appropriate antimicrobial agents to prevent complications leading to severe sepsis and septic shock.[2]
The diagnosis of urosepsis includes both the defining criteria of sepsis and the symptoms and signs of UTI, such as fever, chills, flank pain, burning micturition, urinary retention, and scrotal/prostatic pain. The presence of indwelling catheters could be the underlying risk factor in hospitalized patients and in some debilitating, bedridden patients.[6] Urosepsis evaluation includes clinical examination, ultrasonography of the abdomen and pelvis, routine blood investigations, renal function tests, glomerular filtration rate (GFR), biomarkers like C-reactive protein (CRP), Pro-calcitonin (PCT), urine analysis, urine culture, and at least two sets of blood cultures preferably before starting any antibiotic.[1,6] The number, timing, and the correct volume of the blood are very important in blood cultures to get the possible highest positivity rate.[7] It is observed that only 30% of clinically suspected urosepsis will be blood culture positive.[6] PCT test is reliable and can be used to differentiate sepsis from severe sepsis or septic shock; a level below 0.5 ng/mL usually rules out the presence of severe sepsis/sepsis shock.[6,8,9] Severe sepsis and septic shock need immediate treatment to reduce mortality.[1] The mortality rate of sepsis in the world is 20–40%, and urosepsis accounts for 5–7% of it.[5] The outcome of patients with urosepsis mainly depends on the early etiological diagnosis and appropriate antibiotic therapy.[10]
With this background, this study was conducted to investigate the demographic and clinical characteristics of urosepsis patients admitted to our hospital, their risk factors, bacteriological profile, antimicrobial resistance pattern, and the outcome.
MATERIALS AND METHODS
A prospective observational study was conducted in a tertiary care hospital for a period of 1 year from August 2022 to July 2023 after obtaining ethical clearance (Father Muller Medical College/Father Muller Institutional Ethics committee/336/2022). The sample size was calculated based on the study conducted by Ahmed et al.,[10] in which the prevalence of culture-positive urosepsis was 3.78% (P). Considering a 95% confidence interval and 4% allowable error (e), the sample size was estimated using the formula n = (Z2a/2pq)/d2 = (1.96*1.96*3.78*96.22)/4*4 = 87.
All clinically suspected urosepsis patients were investigated by urine analysis, urine culture, and blood culture with at least two blood culture sets. Urine specimens received in the laboratory were inoculated by semi-quantitative method on Mac Conkey’s agar (HiMedia Laboratories Pvt. Ltd., Mumbai, India), blood agar (HiMedia Laboratories Pvt. Ltd., Mumbai, India), and CHROMagar (HiMedia Laboratories Pvt. Ltd., Mumbai, India), and incubated at 37°C for 24 h. Growth of an isolate was reported as significant if there was pure or predominant growth of bacteria with >105 colony-forming units [CFU]/mL or pure growth of 103–105 CFU/mL with more than 5–6 pus cells/high-power field.[11] Blood cultures were done using a BacT/Alert aerobic culture bottle (bioMérieux, France) and incubated at 37°C for 5 days. Blood culture bottles flagged positively were subcultured on blood agar and MacConkey’s agar. Bacterial identification was done by biochemical reactions and matrix-assisted laser desorption ionizing – time-of-flight mass spectrometry (MALDITOF-MS, Bruker Daltonics), and antimicrobial susceptibility was done by conventional Kirby-Bauer’s disk diffusion method and reported as per Clinical and Laboratory Standards Institute (CLSI) guidelines.[12] The susceptibility results of multidrug-resistant organisms were cross-checked and confirmed by BD Phoenix™ automated identification and susceptibility system. Quality control for Kirby-Bauer’s disk diffusion test, MALDITOF-MS, and BD Phoenix™ automated identification was done using Staphylococcus aureus ATCC® 25923, Escherichia coli ATCC® 25922, and Pseudomonas aeruginosa ATCC® 27853 according to CLSI guidelines.[12]
Patients with UTI growing identical bacterial isolates simultaneously from both urine and blood cultures were confirmed to have urosepsis. Samples growing the same isolate in repeat cultures from the same patient were excluded from the study. Patients growing bacterial isolates in culture taken after 2 days of hospital admission were considered as hospital-acquired and within 48 h as community-acquired infection.
Patients clinical condition and white blood cell count, GFR, CRP (chemiluminescence, Vitros 5600), and PCT (Chemiluminescence, Vitros 5600) levels were checked and recorded. Sepsis was correlated with CRP levels of 10–100 mg/L and PCT levels of 2–10 ng/mL and severe sepsis with PCT levels >10 ng/mL and CRP >100 mg/L.[13]
Inclusion criteria
Patients of >18 years of age and with an accurate diagnosis of UTI with sepsis were included in the study.
Exclusion criteria
Patients under 18 years of age and patients with sepsis of a different origin than UTI were excluded from the study.
All the demographic details, predisposing conditions of the patients, antibiotic treatment, and prognosis were recorded on an Excel sheet.
Statistical analysis was done using IBM Statistical Package for the Social Sciences Statistics V.23. The categorical variables such as demographic, microbiological, and other laboratory characteristics and clinical outcomes were analyzed and expressed in terms of frequencies and percentages. Continuous variables such as age and length of hospital stay were tested for normality of distribution; continuous variables with normal distribution are expressed in terms of mean ± standard deviation, whereas continuous variables with non-normal distribution are expressed in terms of median. Between group comparisons were done by independent t-test for continuous variables with normal distribution and Mann–Whitney U-test for continuous variables with non-normal distribution. Categorical variables are tested using the Chi-square test or Fisher’s exact test.
RESULTS
A total of 1084 (7.9%) patients were admitted to the hospital with clinical symptoms and urine analysis showing significant pus cells and proteinuria suggestive of UTIs. Although about 158 (14.57%) urinary tract infected patients had signs and symptoms of clinical urosepsis, among them, only 87 (8%), patients grew the identical organism from both urine and blood cultures taken simultaneously, which is about 55.06% of the total clinically suspected urosepsis cases. Only these 87 patients with laboratory-confirmed urosepsis were included for further analysis. Twenty-three patients (26.4%) acquired infection in the hospital, 19 out of 23 were on urinary catheters, and the rest 64 patients (73.6%) were community-acquired.
The mean age of the patients was 61.5 years, with 37/87 (42.42%) being above 65 years. Among them, 43/87 (49.44%) were female and 44/87 (50.57%) were male. The risk factors associated with these cases were mainly diabetes mellitus (72.4%), age above 65 years (42.52%), and chronic kidney disease (24.13%) [Table 1]. Among 87 patients, 58 (66.66%) patients had multiple risk factors (two or more). Thirty-eight patients (43.7%) were presented with acute kidney injury associated with urosepsis.
| Risk factor | Number/percentage (n=87) |
|---|---|
| Diabetes mellitus | 63 (72.41) |
| Age>65 years | 37 (42.52) |
| Chronic kidney disease | 21 (24.13) |
| Renal caliculi | 12 (13.79) |
| Urinary catheter | 19 (21.8) |
| Benign prostate hyperplasia | 5 (5.74) |
Gram-negative bacilli (91.95%) were isolated more frequently, with E. coli (68.96%) being the most common organism, followed by Klebsiella pneumoniae (20.68%) [Figure 1]. Only 20 % of Enterobacteriaceae (16/80), including two Enterobacter cloacae isolates, were susceptible to third-generation cephalosporins. Sixteen (18.4%) isolates, including 11 K. pneumoniae and 5 E. coli isolates, were carbapenem-resistant Enterobacteriaceae (CRE) [Figure 2]. A significant association is seen between non-E. coli Gram-negative bacilli with hospital acquired urosepsis (P = 0.002) indwelling urinary catheter (P = 0.004) and carbapenem resistance (P = 0.000) [Table 2].
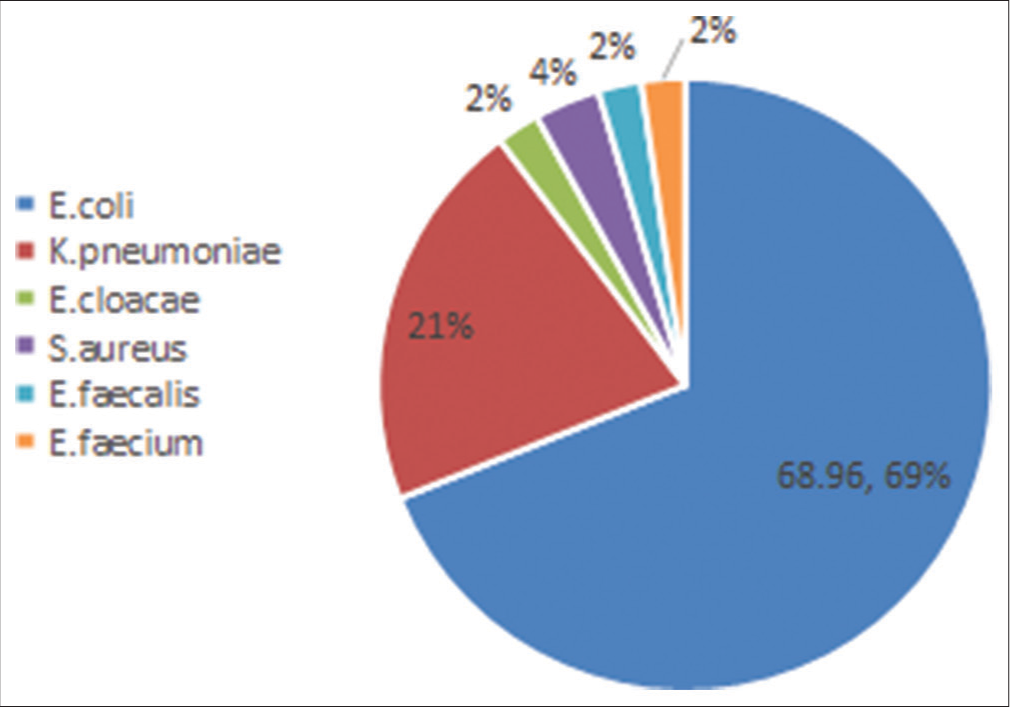
- Distribution of organisms isolated from urosepsis patients.
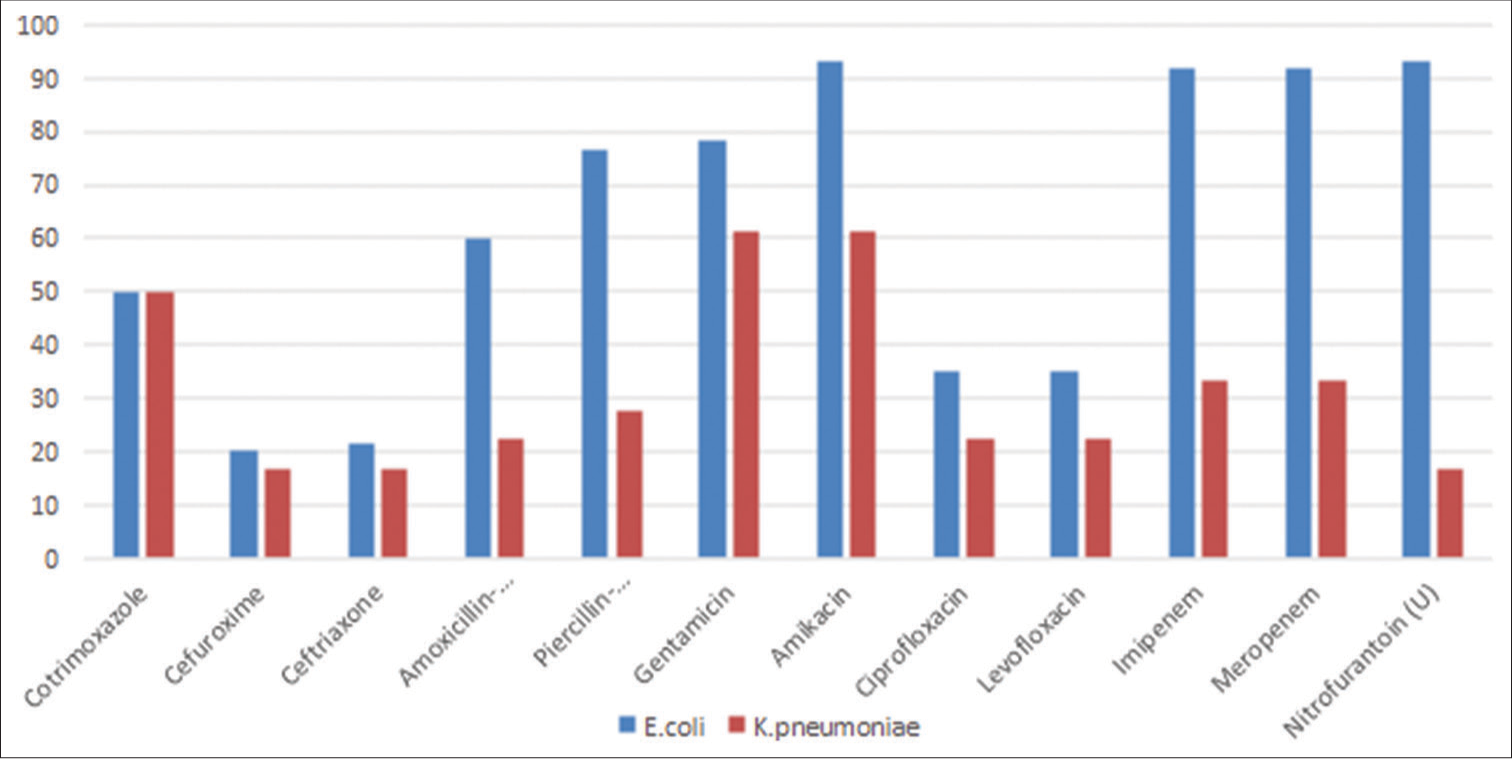
- Antimicrobial susceptibility of most common Gram-negative organisms.
| Characteristics | All patients (87) | E. coliurosepsis 60 (69%) | Non E. coli urosepsis 27 (31%) | P-value |
|---|---|---|---|---|
| Male | 44 | 28 (46.7%) | 16 (59.3%) | 0.277 |
| Age (mean±SD in years) | 61.5 years SD 14.34 years | 61.9 year SD 15.1 years | 60.7 years SD 12.7 years | 0.737 |
| Length of hospital stay – median | 9 days | 9 days | 10 days | 0.426 |
| Community onset (%) | 64 (73.6) | 50 (83.3) | 14 (51.9) | 0.002 |
| BPH (%) | 5 (5.7) | 3 (5) | 2 (7.4) | 0.644 |
| Diabetes mellitus (%) | 63 (72.4) | 43 (71.7) | 20 (74.1) | 0.816 |
| Chronic kidney disease (%) | 21 (24.1) | 15 (25.0) | 6 (22.2) | 0.779 |
| Acute kidney injury (%) | 38 (43.7) | 30 (50) | 8 (29.6) | 0.076 |
| Immunosuppression (%) | 2 (2.3) | 2 (3.3) | 0 | 0.337 |
| Catheter in situ (%) | 19 (21.8) | 8 (13.3) | 11 (40.7) | 0.004 |
| Carbapenem resistance (%) | 16 (18.4) | 5 (8.3) | 11 (40.7) | 0.000 |
| ICU admission (%) | 50 (57.5) | 34 (56.7) | 16 (59.3) | 0.821 |
| Death (%) | 20 (23.0) | 15 (25) | 5 (18.5) | 0.506 |
E. coli: Escherichia coli, ICU: Intensive care unit, SD: Standard deviation, BPH: Benign prostatic hyperplasia
Most of the isolates were susceptible to amikacin (93.33% of E. coli, 61.11% of K. pneumoniae, and two strains of E. cloacae). About 93.33% of urinary E. coli isolates were susceptible to nitrofurantoin, but the susceptibility rate in K. pneumoniae was only 16.66%, and both strains of E. cloacae were resistant [Figure 2].
Gram-positive organisms (8.04%) were less frequently isolated with one methicillin-resistant S. aureus (MRSA). The other two methicillin-sensitive S. aureus (MSSA) strains were also sensitive to fluoroquinolones. All three of them were susceptible to nitrofurantoin and cotrimoxazole. Among Enterococci isolates, both strains of Enterococcus fecalis were susceptible to ampicillin, fluoroquinolones, and nitrofurantoin, whereas both strains of E. faecium were resistant to all three antibiotics. All isolates were susceptible to vancomycin and linezolid [Figure 2].
The most common antibiotic used to treat Gram-negative urosepsis was meropenem (41/80, 51.25%), followed by piperacillin-tazobactam (21/80, 26.25%). Patients with multidrug-resistant Gram-negative organisms were treated with a combination of meropenem and colistin. One patient with MSSA sepsis was treated with ceftriaxone, and the other with piperacillin-tazobactam. Patients with urosepsis caused by MRSA and Enterococci were treated using vancomycin.
The average PCT in urosepsis patients admitted to the intensive care unit (ICU) was 44.67, with a mortality rate of 36%, whereas patients admitted to the ward had an average PCT of 26.36, with a mortality rate of 5.4%. Overall, the mortality rate among urosepsis cases was 23% [Table 3].
| Unit | Number (Percentage) | Average CRP | Average PCT | Mortality rate |
|---|---|---|---|---|
| Number/Percentage | ||||
| ICU | 50 (57.47) | 195.55 | 44.67 | 18 (36) |
| Ward | 37 (42.53) | 174.00 | 26.36 | 02 (5.4) |
| Total | 87 (100) | NA | NA | 20 (23) |
CRP: C Reactive Protein, PCT: Procalcitonin, ICU: Intensive care unit
DISCUSSION
Urosepsis is the sepsis that originates from UTI and it accounts for approximately 30% of all sepsis cases.[14] Identifying sepsis on time and treating with appropriate antibiotics in the initial hours of sepsis is very important for better outcome. The pathogenicity of the infecting organism and the patient’s immune status along with the risk factors decide the course and the severity of sepsis.[6] The most common complication of sepsis is septic shock with a mortality rate of 20–40% both in community acquired and hospitalized patients.[14]
In this study, we found 8% of UTIs developing urosepsis which is slightly higher compared to studies done by Ahmed et al. (3.78%) and Bijou et al. (3.8%).[10,15] For this calculation, we included only inpatients admitted with UTI or developed during hospital stay, into account where as other studies have included all UTI cases. Laboratory confirmed urosepsis with blood culture growth that was obtained only in 55.05% of clinically suspected cases which could due to antibiotic use before the blood cultures or less volume of blood collected for blood culture.
As the age advances, urological comorbidities such as those with indwelling catheter or benign prostate hyperplasia can be expected to become more common and so the incidence of urosepsis.[3] In this study, mean age of the patients was 61.5 years with 42.42% above 65 years which is concordant with studies done.[10,15] The most common risk factor associated with urosepsis was type 2 diabetes mellitus (72.4%) followed by patients with chronic kidney disease (24.1%). Similar findings were observed in Sharma and Duggal and Bijou et al.[13,15] The risk is multiplied with hospitalization with prolonged used of urinary catheter and when there are multiple predisposing conditions. All our patients with hospital acquired urosepsis had urinary catheter in situ showing significant association with non-E. coli urosepsis (P = 0.004).
In urosepsis, as opposed to other type of sepsis, the most commonly isolated pathogen is E. coli, followed by other Enterobacteriaceae with multiple drug-resistant bacteria accounting up to 45%.[10,15,16] In the present study, Gram-negative urosepsis was predominant with E. coli being the most common organism followed by K. pneumoniae. A study conducted by Qiang et al. also found E. coli as the most common causative organism for urosepsis followed by Proteus.[5] We found 43.75% multidrug resistant Gram-negative organisms with resistant to three or more classes of antibiotics mainly resistant to third-generation cephalosporins and fluoroquinolones.
It is of great concern that, we found 18.4% of our Gram-negative isolates were also resistant to carbapenems (CRE) which is the drug of choice for the treatment of urosepsis patients. Compared to E. coli urosepsis, carbapenem resistance was significantly higher in non- E. coli urosepsis specially in K. pneumoniae isolates. At present, there is rapid increase carbapenem resistance in Gram-negative bacilli with higher mortality due to lack of effective antibiotics. Prabhala et al. conducted study in a tertiary care hospital in Mumbai which showed that K. pneumoniae (59.9%) was the most common carbapenem-resistant isolate among all clinical samples.[17] In our study, overall susceptibility of Gram-negative bacilli for amikacin was 86.25% as good as meropenem, so it can be given in combination but has a disadvantage of nephrotoxicity. Our study findings also showed that nitrofurantoin can still be used for uncomplicated community acquired UTI.
It was noticed that there was a direct correlation between the PCT levels and poor clinical out-come of the patients with an average PCT 44.67 ng/mL in patients with severe sepsis with a mortality rate of 36%. Similar findings were observed in other studies.[10,15] Higher PCT levels were observed in Gram-negative sepsis patients with ICU admission and high mortality in our study. PCT levels can be used for the early detection of sepsis and the probable causative organisms, and thus, it can help in choosing the right antibiotic.[13] High mortality was observed in patients with multiple risk factors and in patients with delayed initiation of antibiotic therapy in our study.
Early detection of urosepsis and appropriate antibiotic therapy is very essential to reduce mortality. Since there is high resistance to third-generation cephalosporins and fluoroquinolones, beta-lactam/beta-lactam inhibitor or a carbapenem is necessary for the initial empirical therapy.[14,18] Carbapenem resistance should be in mind if the patient is not improving or worsening of sepsis for which combination of carbapenem with amikacin, colistin, or ceftazidime avibactum can be used. The detection of type of carbapenem resistance will be more useful in deciding antibiotic for definitive therapy.[19] Future studies are required to know the effective antibiotic or combination of antibiotic regimen for the treatment of patients with CREs. Strict antimicrobial stewardship polices and infection prevention and control practices should be in place to combat this threat of multidrug-resistant organisms.
Strengths
It is a prospective observational study and comprehensive data collection of clinical parameters like risk factors, duration of hospital stay, antibiotic treatment, and outcome were done and analyzed.
Limitations
There was no control group (UTI with no sepsis) to compare the risk factors and biomarkers which can, further, improve the diagnosis and help clinicians in better management.
CONCLUSIONS
Urosepsis remains a severe condition with high mortality rate. Gram-negative bacteria, mainly E coli, are the most frequent organism causing urosepsis. Early recognition of symptoms and signs of urosepsis with rapid antibiotic treatment may reduce the mortality. Serum PCT levels can be used for the early detection of sepsis as well as the probable causative organism and thus help in the initiation of antibiotic therapy. Meropenem can still be used as the empirical therapy with careful observation of patients in view of occurrence of carbapenem-resistant Enterobacteriaceae. A comprehensive approach with a team comprising of intensive care specialists, urologists, radiologists, and microbiologists are very important for an effective and rapid management of urosepsis.
Ethical approval
The research/study approved by the Institutional Review Board at Father Muller Medical College, number FMMC/FMIEC/336/2022, dated June 18, 2022.
Declaration of patient consent
Patient's consent not required as patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- The Third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315:801-10.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and management of sepsis. Clin Med (Lond). 2018;18:146-9.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34:15-21.
- [CrossRef] [PubMed] [Google Scholar]
- European Society of infections in urology. Prevalence of hospital-acquired urinary tract infections in urology departments. Eur Urol. 2007;51:1100-11.
- [CrossRef] [PubMed] [Google Scholar]
- Prognosis risk of urospepsis in critical care medicine: A prospective observational study. Biomed Res Int. 2016;2016:9028924.
- [CrossRef] [PubMed] [Google Scholar]
- Urospesis-etiology, diagnosis, and treatment. Dtsch Arztebl Int. 2015;112:837-48.
- [CrossRef] [PubMed] [Google Scholar]
- Principles and procedures for blood cultures; approved guideline In: CLSI document M47-A (2nd ed). Wayne, PA: Clinical and Laboratory Standards Institute; 2022.
- [Google Scholar]
- Prevention, diagnosis, therapy and follow-up care of sepsis: 1st revision of S-2k guide lines of the German Sepsis Society (Deutsche Sepsis-Gesellschaft e. V. (DSG)) and the German interdisciplinary association of intensive care and emergency medicine (deutsche interdisziplinare vereinigung fur intensiv-und notfallmedizin (DIVI)) Ger Med Sci. 2010;8:Doc14.
- [Google Scholar]
- Procalcitonin for early diagnosis and differentiation of SIRS, sepsis, severe sepsis, and septic shock. Intensive Care Med. 2000;26:148-52.
- [CrossRef] [Google Scholar]
- Clinicolaboratory profile and outcome of patients with urosepsis at a tertiary care centre in southern India. J Clin Diagn Res. 2021;15:14-7.
- [CrossRef] [Google Scholar]
- A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2018 update by the infectious diseases society of America and the American society for microbiology. Clin Infect Dis. 2018;67:e1-94.
- [CrossRef] [PubMed] [Google Scholar]
- Performance standards for antimicrobial susceptibility testing In: CLSI Supplement M100 (32nd ed). Wayne, PA: Clinical and Laboratory Standards Institute; 2022.
- [Google Scholar]
- Role of procalcitonin, IL-6 and C-reactive protein in suspected cases of sepsis. Indian J Pathol Microbiol. 2019;62:578-81.
- [CrossRef] [PubMed] [Google Scholar]
- Therapeutic challenges of urosepsis. Eur J Clin Invest. 2008;38:45-9.
- [CrossRef] [PubMed] [Google Scholar]
- Characteristics of blood stream isolates in urosepsis from a tertiary care hospital. Int J Curr Microbiol App Sci. 2016;5:424-31.
- [CrossRef] [Google Scholar]
- Prevalence and molecular characterization of carbapenem resistant gram-negative bacilli in a tertiary care hospital in Mumbai. IP Int J Med Microbiol Trop Dis. 2023;9:150-4.
- [CrossRef] [Google Scholar]
- Treatment guidelines for antimicrobial use in common syndromes (2nd ed). New Delhi, India: ICMR; 2019.
- [Google Scholar]
- Current and emerging treatment options for multidrug resistant Escherichia coli urosepsis: A review. Antibiotics (Basel). 2022;11:1821.
- [CrossRef] [PubMed] [Google Scholar]






