Translate this page into:
Antimicrobial resistance a continued global threat to public health – A perspective and mitigation strategies
*Corresponding author: Laishram Shantikumar Singh, Department of Microbiology, Assam Down Town University, Guwahati, Assam, India. sk1laishram@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Baruah J, Shantikumar Singh L, Salvia T, Sarma J. Antimicrobial resistance a continued global threat to public health – A perspective and mitigation strategies. J Lab Physicians. 2024;16:429-40. doi: 10.25259/JLP_24_2024
Abstract
Antimicrobial resistance (AMR) stands as an imminent menace to global public health, demanding meticulous scrutiny. The speedy expansion of resistant bacteria worldwide jeopardizes the effectiveness of antibiotics, which have altered medicine and saved several lives. The paradigm shifts from the antibiotic era to the era of resistance, particularly within Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species bacteria, which unfolds a pivotal narrative. Categorizing pathogens based on priority illuminates the critical imperative for novel antibiotics. An exhaustive exploration into bacterial resistance mechanisms unveils the intricacies of extended-spectrum cephalosporins resistance, multi-drug resistance, fluoroquinolone resistance, and carbapenem resistance. The complexity intensifies as these pathogens manipulate drug targets, enzymatically neutralize antibiotics, alter outer membrane permeability, and engage in active efflux. The paper discerns the grim repercussions of “Difficult-to-treat resistance,” accentuating elevated mortality rates. A focused analysis of public health, illustrated through the prism of the healthcare landscape, delineates the challenges arising from rampant antibiotic usage, healthcare disparities, and prevailing practices. To address AMR effectively, a comprehensive strategy is imperative. Innovative interventions, alternative treatments, and physicochemical methods are also contributing effectively. Similarly, systematic approaches, including national action plans, antibiotics, and stewardship, are crucial. This scientific exposition underscores the urgency of global collaboration, innovative interventions, and organized strategies to effectively counteract AMR, safeguarding public health in the face of this imminent threat. In this review, we focus on AMR mechanisms and potential strategies for mitigation.
Keywords
Antimicrobial resistance
Resistance mechanisms
ESKAPE bacteria
Mitigation strategies
INTRODUCTION
Antimicrobial resistance (AMR) constitutes a critical and sensitive challenge, posing a substantial threat to public health. The rise of drug-resistant infections threatens global health as pathogens evolve, rendering antimicrobial drugs ineffective and jeopardizing the once miraculous impact of these lifesaving “miracle drugs.” Urgent global efforts are crucial to tackle AMR and preserve the efficacy of essential medical treatments against infectious diseases.[1] The remarkable discovery of penicillin became the groundbreaking finding that remains one of the most widely recognized examples in the history of medicine until the “Golden era” of antibiotics shadowed due to the emergence of the resistance against front-line antimicrobial agents, including the “wonder drug” penicillin even before its large scale use.[2] The discovery and widespread use of antibiotics have revolutionized modern medicine, earning the mid-20th century the title of the “antibiotic era.” During this period, there was a prevailing belief that infectious diseases could be effectively eradicated by the close of the last century, due to the profound impact of antibiotics in combating infections. However, it has emerged as a critical worldwide issue, leading to the development of multi-drug-resistant (MDR) pathogens.[3] Microorganisms, including bacteria, fungi, viruses, and parasites on encounter with antimicrobial drugs such as antibiotics, antifungals, and antivirals can develop AMR. Consequently, standard treatment approaches are becoming less effective, resulting in greater risks of treatment failure, which, in turn, leads to under-acknowledged consequences such as heightened mortality, morbidity, extended hospital stays, and escalated treatment expenses. An insufficient supply of new antibiotics hampers the ability to keep up with the rise in the prevalence of AMR pathogens. Moreover, the unnecessary global use of antibiotics selectively enhances AMR pathogens, exacerbating health risks.[4] The Infectious Diseases Society of America has identified six different species of bacteria as the threat due to their AMR against commonly prescribed antibiotics, and they are named “ESKAPE,” which includes Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species and their prevalence has led to a substantial rise in healthcare expenses.[5] The threat is so concerning that a joint report by the World Health Organization (WHO), United Nations, and World Organization for Animal Health in 2019 mentioned the failure to take appropriate action against drug-resistant diseases could lead to a staggering 10 million deaths globally each year by 2050. In addition, the economic losses are projected to exceed $100 trillion.[6] This scenario [Box 1] emphasizes the urgent need for effective strategies and interventions to combat AMR and safeguard public health and the global economy. Various strategies have been developed to combat this challenge. Current initiatives, such as the WHO’s Global AMR and Use Surveillance System, prioritize to harmonize global reporting of official national AMR and antimicrobial consumption data for a comprehensive understanding and effective strategies.[7]
|
AMR: Antimicrobial resistance, IDSA: Infectious Diseases Society of America, WHO: World Health Organization, UN: United Nations, WOAH: World Organization for Animal Health
IMPACT OF AMR ON PUBLIC HEALTH
AMR is a serious global health threat, particularly in low- and middle-income countries, contributed by several factors such as overuse of antibiotics, especially self-medication. In addition to this, inadequate access to healthcare services, water, sanitation, and hygiene, as well as limited availability of cost-effective diagnostics and affordable antibiotics, have further contributed to the spread of AMR.[8] In 2019, AMR was associated with nearly 600 thousand deaths in the WHO European region and nearly 5 million deaths globally.[9] Hospitals saw a 15 percent increase in AMR infections in 2020, with 80% of hospitalized COVID-19 patients receiving antibiotics.[10] Cassini et al. estimated a significant burden of AMR infections in the European Union and European Economic Area countries, with nearly 700 thousand cases and over 30 thousand deaths, primarily linked to healthcare facilities.[11] The rise in mortality rates and the impact of AMR have been amplified in high-risk populations, including patients with concurrent non-communicable diseases.[5] The inappropriate use of antibiotics, deviation from medical prescriptions, and non-adherence to recommended treatment guidelines have contributed to the development of AMR.[12] Considering the national scenario, India, as a developing country, combating antibiotic resistance poses unique challenges due to exposure to unhygienic conditions, making the population more susceptible to diseases, including antibiotic-resistant infections. This situation diverts resources, limiting access to medical assistance and exacerbating health issues, which, in turn, weakens immunity and complicates efforts to address AMR.[13] A study conducted in rural communities of Tigiria, India, focused on knowledge, attitudes, and practices related to antimicrobial use and resistance. It revealed that while a substantial number of participants were aware of antimicrobial medicines, a significant percentage bought antibiotics without a prescription and even discontinued antibiotic treatment before completing the full course, contributing to the rise of AMR in India.[14] Antibiotic usage in India remains alarmingly high, with 16.29 billion antibiotic doses sold in 2020. Adult usage has increased from nearly 73% to 77% compared to 2018, in contrast to the decreasing trend in developed countries.[15] The impact of AMR is evident in the country, with over 50 thousand newborns dying from sepsis caused by antibiotic-resistant pathogens each year.[16] By 2050, India could witness 2 million deaths due to AMR, making urgent action crucial to tackle this crisis.[17] This challenge is not solely a healthcare issue but extends to misuse in clinical and agricultural settings as well. Therefore, it highlights the need for a comprehensive approach within the holistic framework of “One Health,” which acknowledges the collaborative endeavor of diverse disciplines working at local, national, and global levels to achieve optimal health for people, animals, and the environment.[18]
AMR IN BACTERIA
Throughout evolution, bacteria have developed sophisticated defense mechanisms to combat threats from competitors, bacteriophages, and predators. Unfortunately, these defense mechanisms also lead to the development of antibiotic resistance, which poses significant challenges for modern medicine. The escalating prevalence of antibiotic-resistant pathogens has resulted in a diminishing number of effective antimicrobial agents for the treatment of infections. Projections suggest that without the development or discovery of novel drugs, the availability of viable antibiotics may be severely compromised by 2050.[19] Recognizing the urgency of novel antibiotics, the WHO published a list of bacteria requiring new drugs and research in 2017. The list included critical priority, high priority, and medium priority groups based on the need for novel antibiotics. Bacteria such as Acinetobacter, Pseudomonas, and various Enterobacteriaceae were classified as a critical priority, while high-priority bacteria included E. faecium, S. aureus, Helicobacter pylori, Campylobacter spp., Salmonella, and Neisseria gonorrhoeae. Streptococcus pneumoniae, Haemophilus influenza, and Shigella spp. were considered as medium priority.[20] The top six pathogens causing deaths related to resistance were identified as Escherichia coli, Pseudomonas, Staphylococcus, Klebsiella, Streptococcus, and Acinetobacter.[21] S. aureus, Mycobacterium tuberculosis, as well as emerging pathogens like A. baumannii are the known families of MDR due to their in vitro resistance to more than one antimicrobial agent. Extensively, drug-resistant bacteria, such as carbapenemase-producing K. pneumoniae and A. baumannii, are resistant to nearly all available antibiotics, leaving only older, less effective treatment options like polymyxins. Whereas in tuberculosis (TB) infections, heteroresistance enables subpopulations of M. tuberculosis to survive and proliferate despite the presence of antibiotics, potentially leading to the development of full drug resistance. Similarly, mono-resistance is also observed in M. tuberculosis, where resistance to a single first-line drug such as isoniazid (INH), rifampicin (RIF), ethambutol, pyrazinamide, or streptomycin becomes a significant challenge in the management of TB. INH-monoresistant TB (HR-TB) is the most prevalent form of drug-resistant TB worldwide. Early diagnosis and appropriately adapted treatment are essential for improving outcomes in HR-TB cases.[22] Minor genetic changes can lower the expression of drug resistance, whereas significant genetic alterations can drive high drug resistance in bacteria. Researchers have categorized antimicrobial-resistant Gram-negative bacteria (GNB) into four phenotypical groups, including extended-spectrum cephalosporins resistant, MDR, fluoroquinolone-resistant, and carbapenem-resistant (CR) based on the Centers for Disease Control and Prevention definition.[23] Another classification termed “Difficult-to-treat resistance” (DTR) was introduced by Kadri et al. for AMR among GNB. DTR pertains to resistance against all first-line agents, including β-lactams and fluoroquinolones, providing valuable insights into the challenges posed by AMR in GNB.[24] The impact of DTR was highlighted when significantly higher 30-day mortality rates in gram-negative bloodstream infections (GNBSI) caused by Acinetobacter species or P. aeruginosa were reported compared to non-DTR GNBSI patients.[25] Notably, most CR Acinetobacter isolates exhibit DTR, while a significant proportion of CR P. aeruginosa remain susceptible to other β-lactams or fluoroquinolones.[25]
MECHANISM OF ANTIBIOTIC RESISTANCE
Exposure to antibiotics creates selective pressure on bacterial strains, leading to the survival and dominance of those with antibiotic resistance mechanisms.[26] AMR is exhibited by various Gram-positive bacteria (GPB) as well as by GNB, including CR A. baumannii, Enterobacteriaceae, Pseudomonas, and ESBL-producing Enterobacteriaceae, Vancomycin-resistant Enterococcus (VRE), methicillin-resistant S. aureus (MRSA), and penicillin-non-susceptible S. pneumoniae, emphasizing the urgent need for new research and antibiotics to combat these bacteria.[20] Microorganisms develop AMR through various mechanisms, both in the environment and clinical settings. In clinical settings, resistance can be acquired through chromosomal gene mutations or the transmission of external resistance genes.[27] The mechanisms of AMR primarily involve drug target alteration, enzymatic drug inactivation, changes in outer membrane permeability, inactivation, and active efflux of antimicrobial compounds.[28] These adaptive strategies allow bacteria to evade the effects of antibiotics and contribute to the growing challenge of AMR in healthcare and environmental settings. Figure 1 shows the diagrammatic representation of various resistance mechanisms. Some major mechanisms of resistance to antimicrobial agents are discussed below.
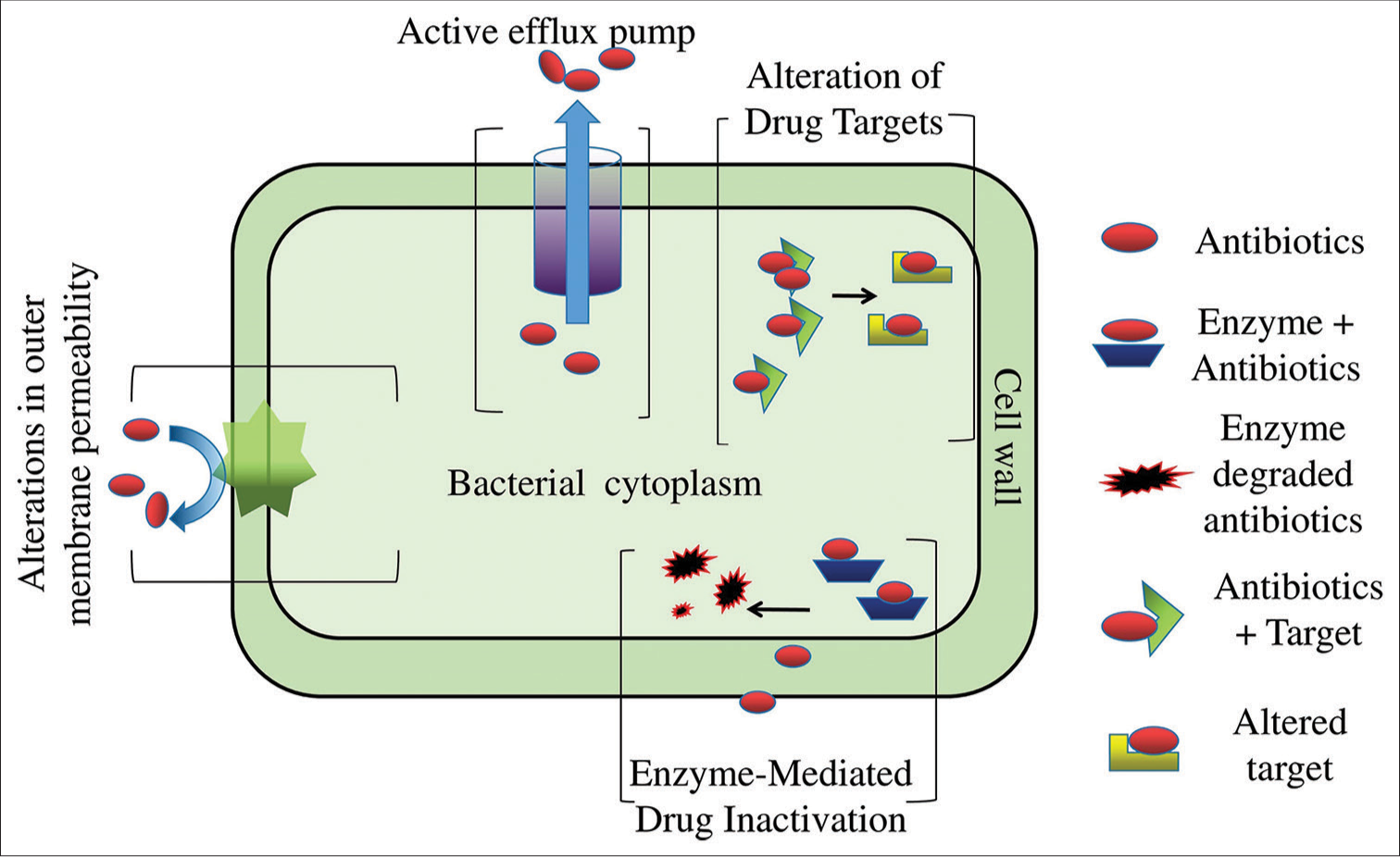
- Illustration of resistance mechanisms exhibited by bacteria against antimicrobial agents.
Alteration of drug targets
Antibiotic resistance in bacteria can be attributed to various structural or compositional changes in the target sites of bacterial cells, as observed in the study by Lade et al.[28] In addition, the presence of separate target protection molecules can prevent antibiotics from effectively binding to their targets, providing a protective shield against the action of antibiotics.[29] For instance, GPB, particularly polymyxin-resistant strains, can develop drug resistance through alterations in antibiotic-binding targets, such as in S. aureus, which acquires resistance by converting its penicillin-binding protein (PBP) to PBP2a, which is encoded by the mecA gene. The recent discovery of mecB, mecC, and mecD genes has further compounded the understanding of antibiotic resistance.[30] PBP2a exhibits low-affinity binding to β-lactam antibiotics and other classes of antibiotics, including macrolides, lincosamides, aminoglycosides, glycopeptides, oxazolidinones, and lipopeptides.[27] The emergence of antibiotic resistance has posed challenges to the use of vancomycin and clindamycin in complicated urinary tract infections and skin-soft-tissue infections caused by MRSA. The emergence of vancomycin-resistant S. aureus and clindamycin-resistant MRSA has significantly reduced the therapeutic efficacy of these antibiotics.[28,31] Enterococci utilize van genes to modify the D-Ala-D-Ala dipeptide at the C-terminus of the translocated pentapeptide to significantly reduce the binding affinity of vancomycin, rendering it less effective against Enterococci.[32] In addition, methylation of the 16S or 23S ribosomal RNA (rRNA) can lead to the loss of efficacy of antibiotics targeting ribosomes, as it disrupts binding affinity by causing steric clashes.[33] To achieve the drug resistance target, protection proteins can act through three mechanisms: direct contact to dislodge the antibiotic, allosteric conformational changes in the target to dislodge the antibiotic, or enabling the target to function despite the presence of the bound antibiotic.[34] Antibiotic resistance ATP-binding cassette proteins (F-subtype) can rescue stalled ribosomes by inducing conformational changes in 23S rRNA, leading to antibiotic dissociation.[35] These diverse mechanisms [Box 2] of antibiotic resistance underscore the complexity of the problem and emphasize the need for innovative strategies to combat the challenge effectively.
|
AMR: Antimicrobial resistance, rRNA: Ribosomal RNA, PBP: Penicillin-binding protein
Enzyme-mediated drug inactivation
Bacteria have produced various enzymes as part of their defense mechanisms against antimicrobial drugs, which render these drugs ineffective by inactivating their action. Three examples of such enzymes are β-lactamase, aminoglycoside inactivating enzyme, and aminoglycoside modifying enzyme. These enzymes fall into different categories, namely, hydrolases, passivation enzymes, and modified enzymes, respectively.[30] The mechanisms of resistance may be further exemplified by highlighting the below-mentioned enzymes.
Resistance by β-lactamase enzymes (BLEs)
Gram-positive and Gram-negative bacteria develop resistance against beta-lactam antibiotics (BLAs) primarily by producing beta-lactamases. BLE-producing bacteria include M. tuberculosis, S. aureus, MRSA, Enterobacteriaceae, P. aeruginosa, and A. baumannii.[36] The comparable structure, geometry, and stereochemistry of penicillin and other β-lactam antibiotics to the enzyme-substrate D-Ala-D-Ala dipeptide explain its efficacy in inhibiting PBPs. Through structural mimicry, these antibiotics disrupt the catalytic activity of bacterial transpeptidases, imitating the amide bonds in the substrate.[37] However, β-lactamase enzymes hydrolyze these antibiotics by targeting the sensitive chemical bonds, particularly the β-lactam ring, through a nucleophilic attack and water addition.[38] BLEs are classified into four classes (A, B, C, and D) based on the Ambler classification and hydrolytic mechanisms.[39] Class B enzymes, known as metallo-BLEs (MBLEs), pose a significant global health concern due to their broad spectrum of actions and lack of clinically approved remedies.[39] On the other hand, classes A, C, and D form the family of active-site serine BLEs.[39] Extended-spectrum β-lactamases (ESBLs), a subset of class A enzymes, are capable of hydrolyzing aztreonam and third-generation cephalosporins, posing a significant threat to public health.[39,40] AmpC β-lactamases are enzymes found primarily in GNB, conferring resistance to a broad range of β-lactams, including penicillins and cephalosporins. Unlike ESBLs, AmpCs resist β-lactam inhibitors like clavulanate but are susceptible to newer inhibitors such as avibactam. Chromosomal AmpCs in bacteria like Enterobacter can be inducible or constitutively expressed, leading to resistance during third-generation cephalosporin therapy due to mutations in regulatory genes like ampD. Plasmid-mediated AmpCs (pAmpCs), like CMY-2, have spread globally, complicating treatment, especially in Enterobacterales. Although pAmpCs and ESBLs rarely co-exist, however when they do, it poses significant clinical challenges. Chromosomal AmpC producers are linked to hospital-acquired infections, while plasmid-mediated producers are more common in community-acquired infections. Class A β-lactamase BlaC in M. tuberculosis hydrolyzes the β-lactam ring through nucleophilic attack by a serine residue, rendering the drug inactive. Unlike other β-lactamases, BlaC hydrolyzes all β-lactam classes, including carbapenems, which are typically resistant to β-lactamases in other bacteria.[41] In addition, β-lactamase inhibitors like clavulanic acid are less effective against BlaC than other class A enzymes.[42] Production of β-lactamases is a concerning challenge [Box 3] in combating AMR.
|
GPB: Gram-positive bacteria, GNB: Gram-negative bacteria, BLAs: Beta-lactam antibiotics, BLEs:Beta-lactamase enzymes, BlaC:Beta-lactamase chromosomally encoded gene, SBLEs: Serine BLEs
Aminoglycosides resistance mechanism
Aminoglycosides have been used to treat a wide spectrum of severe infections caused by both Gram-positive and Gram-negative bacteria. However, various aminoglycoside resistance mechanisms have been acquired by bacteria, such as the production of aminoglycoside-modifying enzymes, enhanced expulsion by efflux pumps, reduced drug permeability, and reduced binding affinity to the target nucleotide in the 16S rRNA which includes post-transcriptional alterations to 16S rRNA facilitated by 16S rRNA methyltransferase enzymes (16S-RMTases).[43] Among these, 16S-RMTases stands out as a critical mechanism of aminoglycoside resistance. The catalysis of 16S-RMTase leads to the addition of a methyl (CH3) group from S-adenosine methionine to specific residues at the A site of 16S rRNA, significantly reducing the binding ability of methylated 16S rRNA to aminoglycosides and resulting in extensive and high-level resistance against various aminoglycosides.[41] Acquired 16S-RMTase fall into two groups based on their methylation target 16S rRNA: G1405 or A1408. G1405 methylation imparts resistance to 4,6-disubstituted 2-deoxystreptamine (DOS) aminoglycosides but not to 4,5-disubstituted 2-DOS. Notable examples include ArmA and RmtA through RmtH for G1405 methylation whereas NpmA stands as the sole identified acquired A1408 16S-RMTase that confers resistance to both 4,5- and 4,6-disubstituted 2-DOS aminoglycosides.[44] In M. tuberculosis, aminoglycoside resistance often involves rrs gene mutations that affect drugs such as kanamycin and amikacin.[45] Whereas in non-tuberculous mycobacteria, the primary mechanism of acquired resistance to aminoglycosides is due to mutations in the 16S rRNA gene. A mutation at position 1408 in nontuberculous mycobacteria the rrs gene is linked to high-level aminoglycoside resistance in species such as Mycobacterium chelonae and Mycobacterium abscessus.[46] These reports highlight the importance of understanding and addressing aminoglycoside resistance mechanisms [Box 4].
|
AMR: Antimicrobial resistance, rRNA: Ribosomal RNA, RMTases: rRNA methyltransferases, rrs:Streptomycin resistant 16S rRNA gene
Alterations in outer membrane permeability
β-lactam antibiotics generally penetrate the outer membrane of GNB through hydrophilic channel proteins, primarily porins, which facilitate the diffusion of small molecules including nutrients and antibiotics. In P. aeruginosa, various resistance mechanisms have been identified, among which intrinsic resistance, particularly the absence or modification of the outer membrane porin OprD, is extensively studied. OprD is a substrate-specific porin that mediates the uptake of carbapenems, such as imipenem and, to a lesser degree, meropenem.[47] However, loss or inactivation of OprD leads to reduced susceptibility to imipenem and, to a lesser extent, meropenem in P. aeruginosa.[47] This loss of function can result from mutations, deletions, or nucleotide substitutions in the oprD gene. Chromosomal mutations that inactivate OprD reduce carbapenem binding to their target proteins, such as PBPs. The impact of OprD inactivation is more pronounced for imipenem than for other carbapenems such as doripenem, meropenem, and ertapenem. Therefore, mutations that affect porin channels or reduce their expression contribute to reduced susceptibility to β-lactams in bacteria, with low outer membrane permeability playing a crucial role in its intrinsic resistance.[48] Furthermore, alterations in the constricting loops of porin channels can influence the diffusion of polar drugs and mutations in these regions selectively obstruct antibiotic passage.[49] The co-existence of carbapenemases and OprD loss complicates diagnostics and limits treatment options. MspA, the major porin of Mycobacterium smegmatis, plays a crucial role in the uptake of hydrophilic antibiotics. Studies have shown that deleting the mspA gene significantly increases resistance to various agents,[50] indicating the importance of porin channels in uptake of drugs or inducement of resistance [Box 5].
|
AMR: Antimicrobial resistance, OprD:outer membrane porins
Active efflux mechanism
The efflux pumps or transmembrane protein complexes are found in clinically significant bacteria. Active efflux is alternatively recognized as the efflux pump system or drug pumping system. The insufficient drug concentration allows bacteria to withstand the impact of the medication, causing drug resistance.[30] Efflux transporters can facilitate the movement of diverse antibiotics out of bacterial cells and the most significant efflux transporters are major facilitator superfamily, ATP-binding cassette, multi-drug and toxic compound extrusion, small multi-drug resistance, and resistance-nodulation-division (RND).[27] RND efflux system falls in the intrinsic resistance mechanism and is the most significantly prevalent superfamily. The primary RND efflux pumps include MexAB-OprM, MexCD-OprJ, MexEF-OprN, and MexXY/OprM (OprA). This efflux pump includes proteins such as AcrB in E. coli, MexB in P. aeruginosa, and MtrD in N. gonorrhoeae, as well as CmeB and CusA, which provide resistance to heavy metals.[51] The three major components of these pumps are as follows: (i) Efflux Transporter is an inner membrane protein of the bacterial cell responsible for capturing the substances (e.g., antibiotics) from inside the cell or from the inner membrane itself. (ii) Outer Membrane Channel is the pathway that expels captured substances from the cell, moving them from the inside to the outside environment. (iii) Accessory Protein (Periplasmic) connects the efflux transporter and the outer membrane channel that ensures proper communication and coordination between the two, allowing the substances to be efficiently transported out of the cell. Efflux pumps, such as the MmpL and MmpS systems, contribute to intrinsic resistance in M. tuberculosis by actively expelling drugs. Active efflux pump is also involved in gram positive bacteria in inducible antibiotic resistance. Their proficiency in expelling antibiotics has turned them into an effective mechanism for AMR [Box 6].[52]
|
AMR: Antimicrobial resistance, MFS: Major facilitator superfamily, ABC: ATP-binding cassette, MATE: Multi-drug and toxic compound extrusion, SMR: Small multi-drug resistance, RND: Resistance-nodulation-division
MITIGATION STRATEGIES FOR AMR
The challenge of AMR directs for novel and innovative strategies to mitigate its impact on public health. Routine surveillance by the WHO helps to monitor AMR trends in various pathogens, enabling targeted interventions and effective disease management.[7] In-fact, developing new-generation drugs that target surface-exposed features like β-barrel assembly machinery in GNB shows promising result in combating AMR.[53] The identification of potent inhibitors like indole-2-carboxylates for MBLEs offers a potential strategy in restoring efficacy of carbapenem antibiotics against drug-resistant GNB.[54] However, the inefficiency of antibiotics against bacteria highlights the urgency to explore alternative treatments. Targeting resistance mechanisms, such as plasmid curing, bacteriophages, and bacteriotherapy, could provide feasible alternatives to combat MDR pathogens.[55] Comprehensive strategies, including drug delivery systems and targeting AMR enzymes, have been reported in addressing the AMR crisis.[56] Previous reports have outlined that the understanding of antibiotic resistance mechanisms, especially in MDR organisms, has led to the various resistance mechanism inhibitors, including efflux pump inhibitors, ribosomal inhibitors, and AMR gene silencers such as the clustered regularly interspaced short palindromic repeats (CRISPR)-Cas system (CRISPR-associated protein system).[56,57] Figure 2 highlights some of the potential strategies to combat AMR.
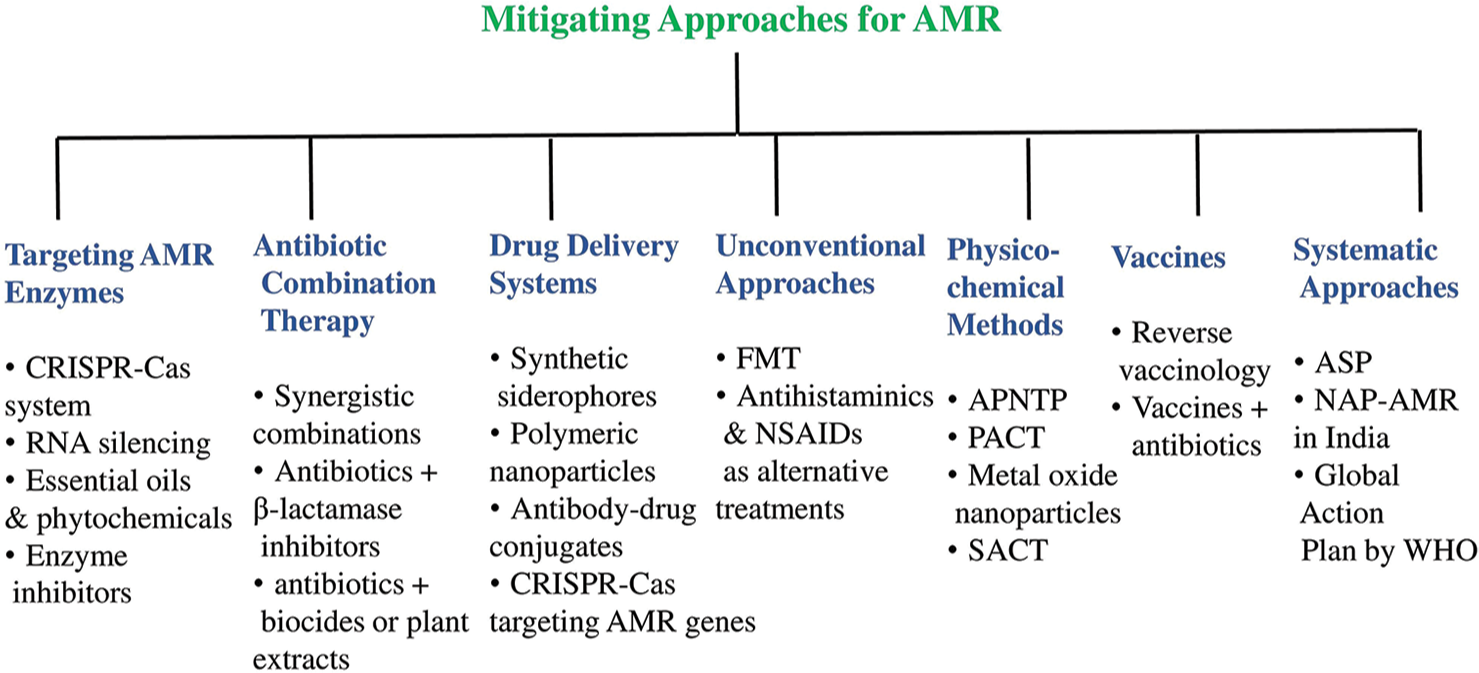
- Some of the potential mitigating strategies for antimicrobial resistance (AMR). CRISPRCas: Clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR associated proteins (Cas) system, RNA: Ribonucleic Acid, FMT: Fecal Microbiota Transplantation, NSAIDs: Non-steroidal anti-inflammatory drugs, APNTP: Atmospheric Pressure Non-Thermal Plasma, PACT: Photoinactivation or photodynamics Antimicrobial Chemotherapy, SACT: Sonodynamic Antimicrobial Chemotherapy, ASP: Antimicrobial Stewardship Program, NAP-AMR: National Action Plan on Antimicrobial Resistance, WHO: World Health Organization.
Targeting AMR enzymes
Several effective approaches have been explored to target AMR enzymes. These approaches include the CRISPR-Cas system, RNA silencing, essential oils, Small Molecules-Improved Chemical Entities, phytochemicals, and enzyme inhibitors.[56,58,59] The CRISPR-Cas system, renowned for gene editing, can target AMR genes in bacteria and disrupt these genes, rendering the bacteria susceptible to antibiotics again. It is a bacterial defense system which utilizes RNA-guided, DNA-encoded, or DNA-targeting mechanisms to defend against the intrusion of bacteria by mobile genetic elements and foreign genetic material, including plasmids and phages. Another promising approach involves RNA silencing, where binding of complementary cis and trans sequences to the regulatory regions of mRNA prevents the synthesis of proteins during translation. Antimicrobial compounds obtained from plants sources as well as the essential oils have been found useful as alternative strategies to control bacterial infections. Essential oils are known to exhibit potent antimicrobial activity, while Small Molecules-Improved Chemical Entities are the novel direct-acting small molecules which may emerge through the improvement of existing antibiotics or by the production of new compounds with innovative targets and mechanisms of action.[55,59] Phytochemicals or secondary metabolites of plants have shown potential as antibacterials both on their own and in combination with other antibacterial agents which are considered as a significant alternative treatment. Enzyme inhibitors are the entity intended to specifically inhibit activity of AMR enzymes, thereby restoring the efficacy of antibiotics. Figure 3 represents various approaches for targeting AMR enzymes.
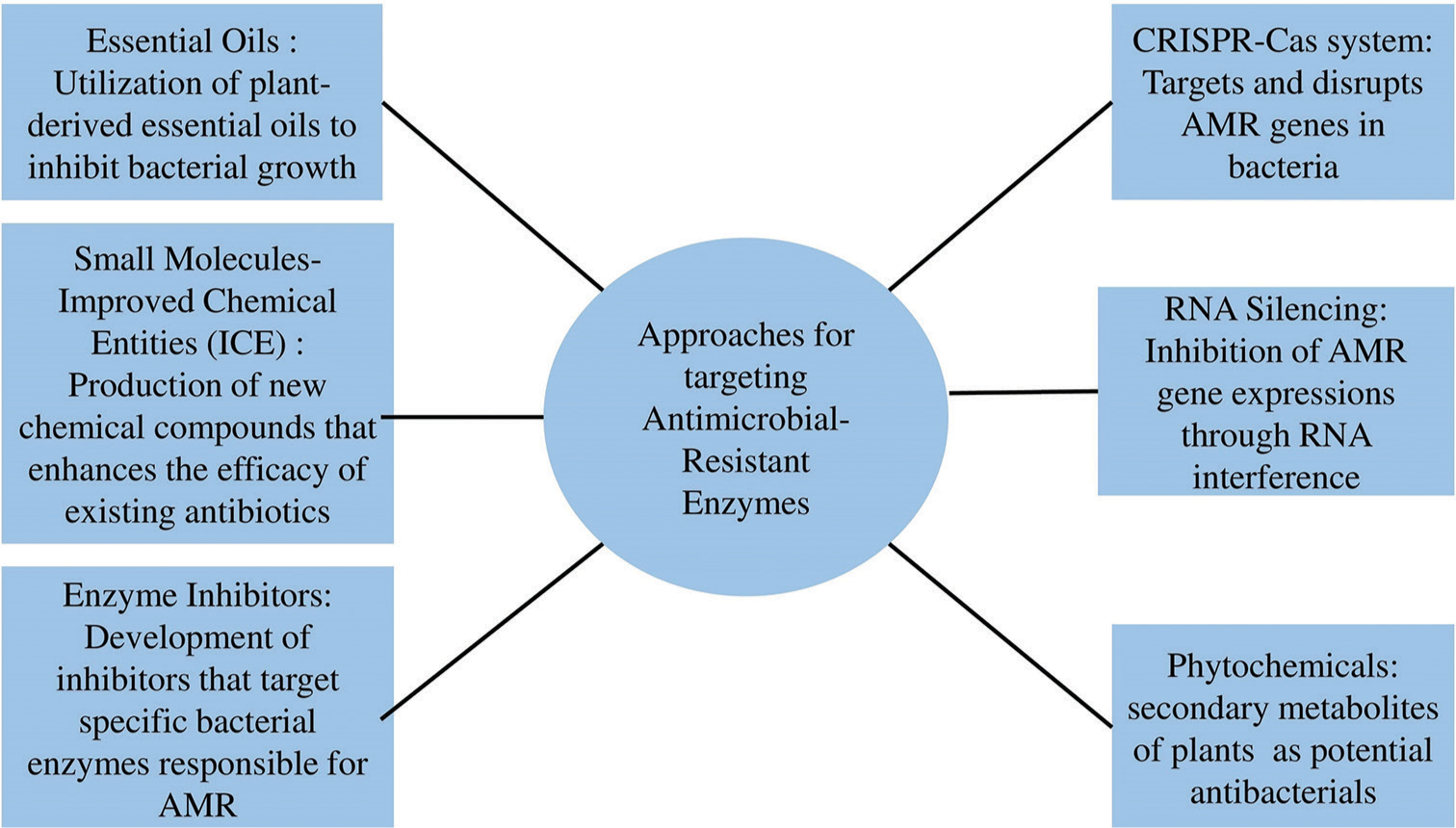
- Various approaches for targeting antimicrobial resistance enzymes. AMR: Antimicrobial resistance.
Antibiotic combination therapy
Antibiotic combination therapy and amalgamating antibiotics in novel regimens embraces to be a valuable approach in combating drug-resistant infections. The concept of antibiotic synergy refers to the amplified effectiveness of antibiotics when combined at the optimal ratio.[60] When certain drugs are judiciously chosen and combined in the accurate proportion, the bactericidal activity becomes synergistic having substantial ability in killing bacteria resistant to antibiotics. Such a therapy has previously been practiced in the treatment of TB, infection by human immunodeficiency virus and also in other non-infective aliments like chemotherapy of cancer. The synergistic amalgamation therapy can be established reasonably for application in patients harmlessly as the component of such medicines is well identified.[60] One well-established example is the combination of a β-lactam antibiotic with an aminoglycoside, which has been extensively utilized to treat various Gram-negative bacterial infections. Another effective approach involves combining antibiotics with β-lactamase inhibitors.[61] β-lactamase inhibitors prevent the breakdown of β-lactam antibiotics by the bacterial enzymes, thus enhancing their efficacy and expanding their spectrum of activity. In recent times, the combination of antibiotics with biocides has also emerged as a successful therapeutic strategy.[62] In TB, meropenem, when combined with clavulanic acid, shows potential for treating susceptible TB as it is effective against non-replicating forms of bacilli, which are challenging to eliminate even with standard first-line drugs such as INH and RIF. Furthermore, a constant and repetitive synergy has been observed by combining various antibiotics with plant extracts, which has increased the inhibitory effects against bacteria compared to using antibiotics alone.[63] Combination therapy is an effective approach, as shown in Figure 4, and can lead to enhance treatment outcomes against resistant bacteria.
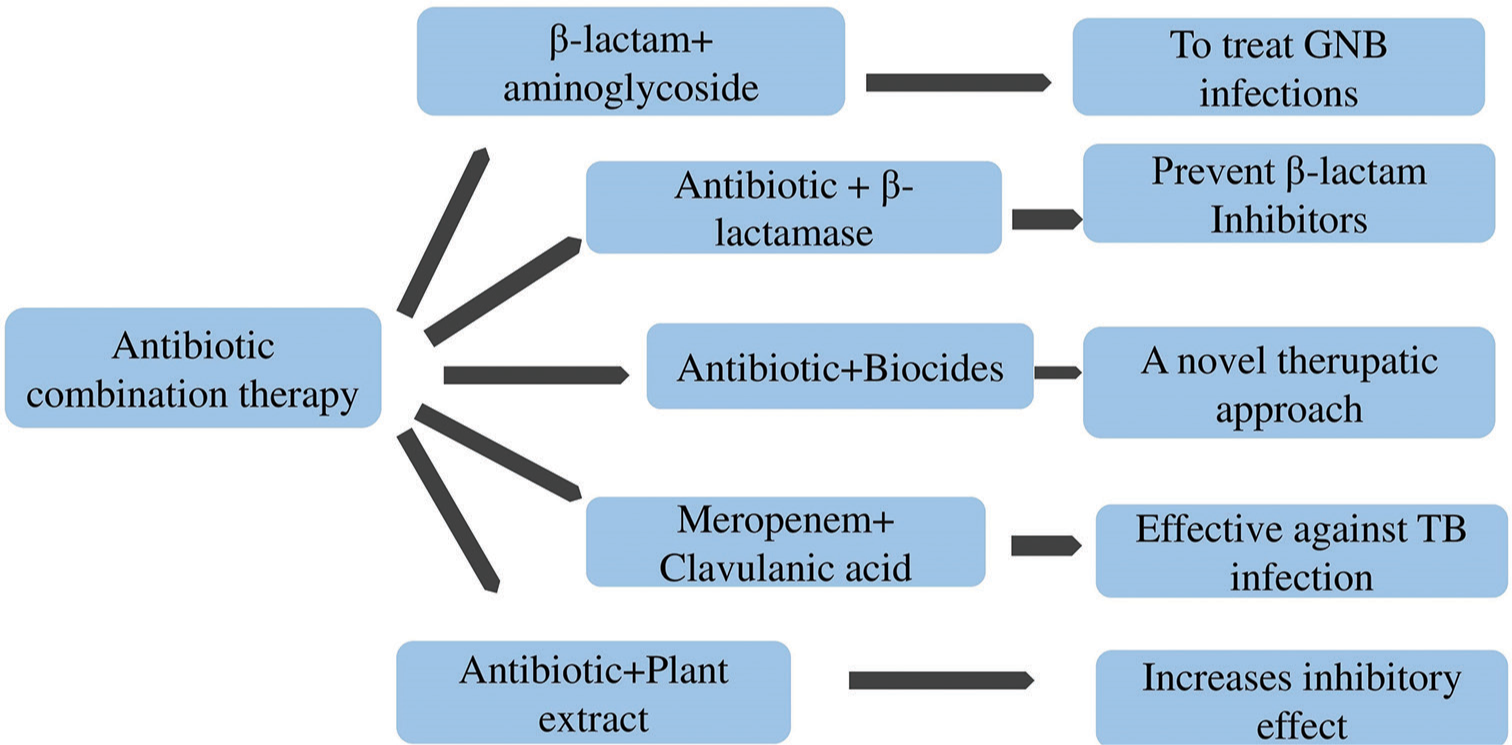
- Combating antimicrobial resistance through antibiotic combination therapy. AMR: Antimicrobial resistance, RNA: Ribonucleic acid, GNB: Gram-negative bacteria, TB: Tuberculosis, CRISPR: Clustered regularly interspaced short palindromic repeats
Drug delivery system
Researchers are actively exploring various approaches to combat AMR bacteria. One promising avenue is the use of drug delivery systems, such as synthetic siderophores and polymeric nanoparticles, which have shown potential in enhancing the effectiveness of antibiotics.[64] Innovative techniques, such as antibody-drug conjugates and CRISPRCas systems, target AMR genes in plasmid with the aim of reducing resistance in the bacteria.[65] Another alternative approach involves antivirulence compounds, like quorum sensing inhibitors, which can disrupt bacterial communication and coordination, thus reducing bacterial pathogenicity.[66]
Unconventional approaches
Researchers have explored various alternative drugs against AMR infections, including antihistaminic, non-steroidal anti-inflammatory drugs, anesthetics, antipsychotics, and cardiovascular drugs. One promising approach is fecal microbiota transplantation (FMT), which has shown high efficacy in treating recurrent Clostridium difficile infections by restoring a healthy gut microbiota.[67] FMT has proven to be effective in displacing VRE particularly when it is the dominant microorganism within the gut microbiota.
Physicochemical methods
Physicochemical methods for combating AMR offer innovative approaches to address drug-resistant microorganisms.[59] One such method is Atmospheric Pressure Non-Thermal Plasma, which effectively targets bacteria, viruses, fungi, and parasites with minimal side effects compared to conventional treatments.[68] Photoinactivation or photodynamic antimicrobial chemotherapy (PACT) is another promising technique that utilizes visible light and photosensitizers to generate reactive oxygen species (ROS), effectively killing bacteria. PACT shows potential in combating AMR with localized application and reduced risk of resistance development.[69] Metal oxide nanoparticles, including Silver, Iron (II, III) oxide, Titanium dioxide, Copper oxide, and Zinc oxide, demonstrate potent antibacterial effects through ROS generation. However, their use requires caution due to potential environmental impact and co-selection risks.[70] Sonodynamic antimicrobial chemotherapy is another physicochemical method that utilizes ultrasound and sonosensitizers to efficiently and precisely kill microorganisms while minimizing side effects.[71] Physicochemical strategies are considered as an advanced and effective mitigating approach [Figure 5].
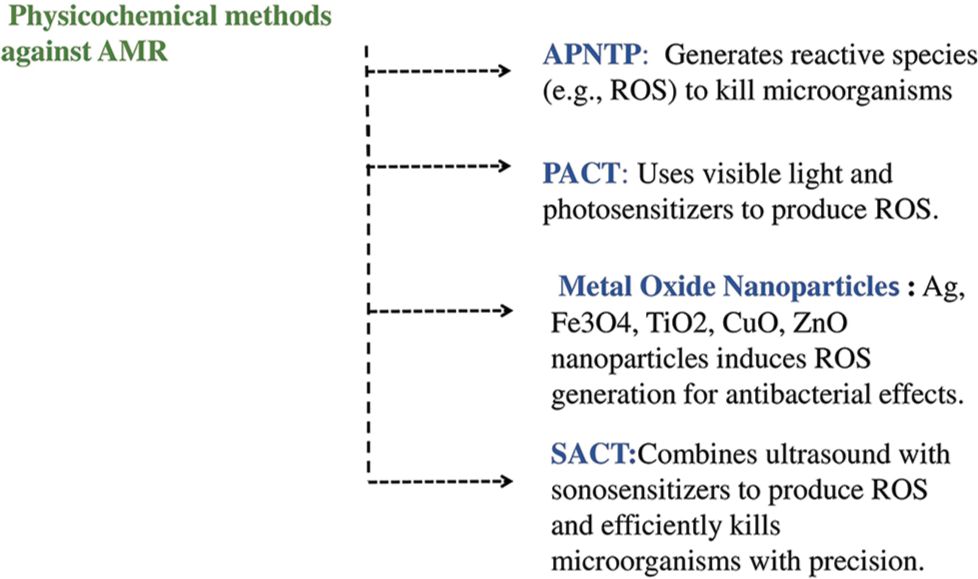
- Advanced physicochemical strategies as mitigating approach for antimicrobial resistance (AMR). APNTP: Atmospheric Pressure Non-Thermal Plasma, ROS: Reactive Oxygen Species, PACT: Photoinactivation or photodynamics Antimicrobial Chemotherapy, Ag: Silver, Fe3O4: Iron (II,III) oxide, TiO2: Titanium dioxide, CuO: Copper oxide, ZnO: Zinc oxide, SACT: Sonodynamic Antimicrobial Chemotherapy.
Vaccines
Vaccines directly reduce resistant pathogens and limit AMR spread. Innovative approaches such as reverse vaccinology and combining vaccines with antibiotics can target resistant strains.[72] Vaccines affect resistance pathogens directly by lowering the incidence of infection and indirectly by lowering the spread of AMR-resistant strains among non-resistant species.[56] Vaccination complements antibiotics in curbing AMR.
Systematic approaches
In September 2016, Ministry of Health and Family Welfare of India has established the National Action Plan on AMR (NAP-AMR) which is a comprehensive strategy to combat AMR, through collaborative efforts of three technical bodies, namely, Intersectoral Coordination Committee, Technical Advisory Group and Core Working Group. The WHO’s Global Action Plan on AMR, which was approved by the World Health Assembly in 2015, serves as a major foundation for the NAP-AMR.[73] Antimicrobial Stewardship (AMS) is crucial for ensuring antibiotics that are used only when necessary. The WHO advocates AMS programs to promote responsible antibiotic use, emphasizing the correct dose, reason, and duration of treatment. The introduction of the Antimicrobial Stewardship Program (ASP) was well-received for its systematic approach. However, the widespread use of broad-spectrum antibiotics during the COVID-19 pandemic has exacerbated antibacterial resistance, particularly among MRSA and ESBL-producing bacteria. This has transformed healthcare facilities into hubs for MDR organisms. ASP aims to enhance the recognition, implementation, and innovation in antibiotic use, with future priorities in focus. Despite progress, such as expanding ASP to substantial number of secondary hospitals and establishing the Indian Council of Medical Research-AMR Surveillance Network, India faces significant challenges due to limited resources and laboratory capacity.[74] Key achievements include the formation of a network of 20 tertiary care hospitals for AMR surveillance, including 14 government and 6 private institutions[75], which include various initiatives [Box 7], required for harmonious work.
|
ICMR: Indian Council of Medical Research
Combating AMR is a collective endeavor that demands a multifaceted strategy, involving the discovery of new drugs, responsible antibiotic use, improved diagnostics, robust infection prevention, continuous monitoring, and international cooperation to effectively address and prevent the spread of resistance.
CONCLUSIONS
Addressing the global challenge, AMR demands coordinated efforts at local, national, and international levels. Organizations such as the WHO and the World Bank have been actively involved in supporting countries in their efforts to combat AMR. Initiatives like the Global Action Plan on AMR have served as a blueprint for guiding countries in their strategies to tackle this global challenge. In addition to policy-level interventions, research and innovation are crucial components in this crisis. Encouraging investment in the development of new antibiotics and diagnostic tools is also essential as antibiotic effectiveness has been increased by a number of innovative targets and techniques, such as editing, silencing, and inactivation of resistance genes. Moreover, exploring alternative treatments, such as FMT, showcases the importance of unconventional strategies in this challenge. Similarly, Public awareness and education campaigns also play a key role to empower individuals with knowledge about AMR and responsible antibiotic use. AMR is a multifaceted challenge that requires a comprehensive and coordinated response from all sectors of society. From healthcare professionals to policymakers, researchers, and the general public, everyone has a role to play in protecting the effectiveness of antibiotics and safeguarding public health. Through collective action and commitment, the impact of AMR can be mitigated and a healthier future for the coming generations can be secured.
Acknowledgments
The authors are grateful to the authorities of the host institution for their support and encouragement to carry out this work.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Emerging MDR Enterobacteriaceae and the antimicrobial resistance pattern in Indian Scenario: Challenge after COVID-19 pandemic. IJBPAS. 2023;12:60-373.
- [CrossRef] [Google Scholar]
- Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 2010;74:417-33.
- [CrossRef] [PubMed] [Google Scholar]
- Penicillin and the antibiotics revolution global history. Asian J Pharm Res. 2023;13:55-62.
- [CrossRef] [Google Scholar]
- Antimicrobial resistance, mechanisms and its clinical significance. Dis Mon. 2020;66:100971.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and economic impact of antibiotic resistance in developing countries. A systematic review and meta-analysis. PLoS One. 2017;12:e0189621.
- [CrossRef] [PubMed] [Google Scholar]
- Tackling drug-resistant infections globally: Final report and recommendations In: The review on antimicrobial resistance. 2016. p. :1-80. Available from: https://apo.org.au/sites/default/files/resource-files/2016-05/apo-nid63983.pdf [Last accessed on 2024 Feb 12]
- [Google Scholar]
- Global antimicrobial resistance and use surveillance system (GLASS) report. 2021. Available from: https://www.who.int/publications/i/item/9789240027336 [Last accessed on 2024 Feb 12]
- [Google Scholar]
- Antimicrobial resistance: Addressing a global threat to humanity. Plos Med. 2023;20:e1004264.
- [CrossRef] [PubMed] [Google Scholar]
- The burden of bacterial antimicrobial resistance in the WHO European region in 2019: A cross-country systematic analysis. Lancet Public Health. 2022;7:e897-913.
- [Google Scholar]
- Diagnostic and antimicrobial stewardship workforce challenges: A crisis in combating antimicrobial resistance. Antimicrob Steward Healthc Epidemiol. 2023;3:e60.
- [CrossRef] [PubMed] [Google Scholar]
- Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect Dis. 2019;19:56-66.
- [CrossRef] [PubMed] [Google Scholar]
- Tackling the global non-prescription use of antibiotics. Lancet Infect Dis. 2020;20:169-70.
- [CrossRef] [PubMed] [Google Scholar]
- Antimicrobial resistance in the environment: The Indian scenario. Indian J Med Res. 2019;149:119-28.
- [CrossRef] [PubMed] [Google Scholar]
- Perception and determinants leading to antimicrobial (MIS) use: A knowledge, attitude, and practices study in the rural communities of Odisha, India. Front Public Health. 2023;10:1074154.
- [CrossRef] [PubMed] [Google Scholar]
- Sales of antibiotics and hydroxychloroquine in India during the COVID-19 epidemic: An interrupted time series analysis. PLoS Med. 2021;18:e1003682.
- [CrossRef] [PubMed] [Google Scholar]
- Antibiotic resistance-the need for global solutions. Lancet Infect Dis. 2013;13:1057-98.
- [CrossRef] [PubMed] [Google Scholar]
- Antimicrobial resistance: Progress in the decade since emergence of New Delhi metallo-β-lactamase in India. Indian J Community Med. 2019;44:4.
- [CrossRef] [PubMed] [Google Scholar]
- Tackling AMR from a multidisciplinary perspective: A primer from education and psychology. Int Microbiol. 2023;26:1-9.
- [CrossRef] [PubMed] [Google Scholar]
- Multidrug-resistant bacteria and alternative methods to control them: An overview. Microb Drug Resist. 2019;25:890-908.
- [CrossRef] [PubMed] [Google Scholar]
- Antimicrobial resistance in ESKAPE pathogens. Clin Microbiol Rev. 2020;33:e00181-19.
- [CrossRef] [PubMed] [Google Scholar]
- Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet. 2022;399:629-55.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular detection of isoniazid monoresistance improves tuberculosis treatment: A retrospective cohort in France. J Infect. 2022;85:24-30.
- [CrossRef] [PubMed] [Google Scholar]
- Pathogen distribution and antimicrobial resistance among pediatric healthcare-associated infections reported to the National Healthcare Safety Network, 2011-2014. Infect Control Hosp Epidemiol. 2018;39:1-11.
- [CrossRef] [PubMed] [Google Scholar]
- Difficult-to-treat resistance in gram-negative bacteremia at 173 US hospitals: Retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin Infect Dis. 2018;67:1803-14.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of difficult-to-treat resistance in gram-negative bacteremia on mortality: Retrospective analysis of nationwide surveillance data. Clin Infect Dis. 2020;71:487-96.
- [CrossRef] [PubMed] [Google Scholar]
- Overcoming the rising incidence and evolving mechanisms of antibiotic resistance by novel drug delivery approaches-an overview. Adv Drug Deliv Rev. 2022;181:114078.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanisms of antibiotic resistance In: Kudva IT, Cornick NA, Zhang PQ, Nicholson TL, Bannantine JP, Bellaire BH, eds. Virulence mechanisms of bacterial pathogens. United States: John Wiley and Sons; 2016. p. :481-511.
- [CrossRef] [Google Scholar]
- Molecular basis of non-β-lactam antibiotics resistance in Staphylococcus aureus. Antibiotics (Basel). 2022;11:1378.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular mechanisms of antibiotic resistance revisited. Nat Rev Microbiol. 2023;21:280-95.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical perspective of antimicrobial resistance in bacteria. Infect Drug Resist. 2022;15:735-46.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of methicillin-resistant Staphylococcus aureus (MRSA): Updated guidelines from the UK. JAC Antimicrob Resist. 2021;3:dlaa114.
- [CrossRef] [PubMed] [Google Scholar]
- Antimicrobial-resistant CC17 Enterococcus faecium: The past, the present and the future. J Glob Antimicrob Resist. 2019;16:36-47.
- [CrossRef] [PubMed] [Google Scholar]
- Antibiotic resistance by enzymatic modification of antibiotic targets. Trends Mol Med. 2020;26:768-82.
- [CrossRef] [PubMed] [Google Scholar]
- Target protection as a key antibiotic resistance mechanism. Nat Rev Microbiol. 2020;18:637-48.
- [CrossRef] [PubMed] [Google Scholar]
- Structural basis for PoxtA-mediated resistance to phenicol and oxazolidinone antibiotics. Nat Commun. 2022;13:1860.
- [CrossRef] [PubMed] [Google Scholar]
- Old and new beta-lactamase inhibitors: Molecular structure, mechanism of action, and clinical Use. Antibiotics (Basel). 2021;10:995.
- [CrossRef] [PubMed] [Google Scholar]
- β-lactam antibiotics: An overview from a medicinal chemistry perspective. Eur J Med Chem. 2020;208:112829.
- [CrossRef] [PubMed] [Google Scholar]
- Antibiotic resistance by enzyme inactivation: From mechanisms to solutions. Chembiochem. 2010;11:1325-34.
- [CrossRef] [PubMed] [Google Scholar]
- β-lactam antibiotics and β-lactamase enzymes inhibitors, part 2: Our limited resources. Pharmaceuticals. 2022;15:476.
- [CrossRef] [PubMed] [Google Scholar]
- Bacterial resistance to antimicrobial agents. Antibiotics. 2021;10:593.
- [CrossRef] [PubMed] [Google Scholar]
- Novel plasmid-mediated 16S rRNA m1A1408 methyltransferase, NpmA, found in a clinically isolated Escherichia coli strain resistant to structurally diverse aminoglycosides. Antimicrob Agents Chemother. 2007;51:4401-9.
- [CrossRef] [PubMed] [Google Scholar]
- Meropenem-clavulanate is effective against extensively drug-resistant Mycobacterium tuberculosis. Science. 2009;323:1215-8.
- [CrossRef] [PubMed] [Google Scholar]
- Genomic characterization of 16S rRNA methyltransferase-producing Escherichia coli isolates from the Parisian area, France. J Antimicrob Chemother. 2020;75:1726-35.
- [CrossRef] [PubMed] [Google Scholar]
- Functional and structural characterization of acquired 16S rRNA methyltransferase NpmB1 conferring pan-aminoglycoside resistance. Antimicrob Agents Chemother. 2021;65:10-1128.
- [CrossRef] [PubMed] [Google Scholar]
- ubiA (Rv3806c) encoding DPPR synthase involved in cell wall synthesis is associated with ethambutol resistance in Mycobacterium tuberculosis. Tuberculosis (Edinb). 2015;95:149-54.
- [CrossRef] [PubMed] [Google Scholar]
- A single 16S ribosomal RNA substitution is responsible for resistance to amikacin and other 2-deoxystreptamine aminoglycosides in Mycobacterium abscessus and Mycobacterium chelonae. Infect Dis. 1998;177:1573-81.
- [CrossRef] [PubMed] [Google Scholar]
- Coexistence of multidrug resistance mechanisms and virulence genes in carbapenem-resistant Pseudomonas aeruginosa strains from a tertiary care hospital in South India. J Glob Antimicrob Resist. 2018;12:37-43.
- [CrossRef] [PubMed] [Google Scholar]
- Structure, function and regulation of Pseudomonas aeruginosa porins. FEMS Microbiol Rev. 2017;41:698-722.
- [CrossRef] [PubMed] [Google Scholar]
- Porins and small-molecule translocation across the outer membrane of Gram-negative bacteria. Nat Rev Microbiol. 2020;18:164-76.
- [CrossRef] [PubMed] [Google Scholar]
- Multidrug resistance of a porin deletion mutant of Mycobacterium smegmatis. Antimicrob Agents Chemother. 2004;48:4163-70.
- [CrossRef] [PubMed] [Google Scholar]
- Structural and functional diversity of resistance-nodulation-cell division transporters. Chem Rev. 2021;121:5378-16.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular characterization of extended-spectrum beta-lactamases and carbapenemases producing Enterobacteriaceae isolated from North Eastern region of India. J Lab Physicians. 2024;16:245-52.
- [CrossRef] [Google Scholar]
- A ban on BAM: An update on inhibitors of the β-barrel assembly machinery. FEMS Microbiol Lett. 2021;368:fnab059.
- [CrossRef] [PubMed] [Google Scholar]
- Imitation of β-lactam binding enables broad-spectrum metallo-β-lactamase inhibitors. Nat Chem. 2022;14:15-24.
- [CrossRef] [PubMed] [Google Scholar]
- New tools to mitigate drug resistance in Enterobacteriaceae-Escherichia coli and Klebsiella pneumoniae. Crit Rev Microbiol. 2023;49:435-54.
- [CrossRef] [PubMed] [Google Scholar]
- Progress in alternative strategies to combat antimicrobial resistance: Focus on antibiotics. Antibiotics (Basel). 2022;11:200.
- [CrossRef] [PubMed] [Google Scholar]
- Phenotypic detection of ESBL, AmpC, MBL, and their cooccurrence among MDR Enterobacteriaceae isolates. J Lab Physicians. 2022;14:329-35.
- [CrossRef] [PubMed] [Google Scholar]
- CRISPR-based antimicrobials to obstruct antibiotic-resistant and pathogenic bacteria. PLoS Pathog. 2021;17:e1009672.
- [CrossRef] [PubMed] [Google Scholar]
- Essential oils: A natural weapon against antibiotic-resistant bacteria responsible for nosocomial infections. Antibiotics (Basel). 2021;10:417.
- [CrossRef] [PubMed] [Google Scholar]
- Antibiotic combination therapy against resistant bacterial infections: Synergy, rejuvenation and resistance reduction. Expert Rev Anti Infect Ther. 2020;18:5-15.
- [CrossRef] [PubMed] [Google Scholar]
- The latest advances in β-lactam/β-lactamase inhibitor combinations for the treatment of Gram-negative bacterial infections. Expert Opin Pharmacother. 2019;20:2169-84.
- [CrossRef] [PubMed] [Google Scholar]
- Species-specific activity of antibacterial drug combinations. Nature. 2018;559:259-63.
- [CrossRef] [PubMed] [Google Scholar]
- Synergistic combinatorial strategy for combating Antimicrobial Resistance (AMR) in clinical bacteria by combining antibiotics with plant extracts. Fine Chem Eng. 2023;4:1-12.
- [CrossRef] [Google Scholar]
- Drug delivery systems designed to overcome antimicrobial resistance. Med Res Rev. 2019;39:2343-96.
- [CrossRef] [PubMed] [Google Scholar]
- Preclinical and translational pharmacokinetics of a novel THIOMABTM antibody-antibiotic conjugate against Staphylococcus aureus. MAbs. 2019;11:1162-74.
- [CrossRef] [PubMed] [Google Scholar]
- Antivirulence compounds: A future direction to overcome antibiotic resistance? Future Microbiol. 2020;15:299-301.
- [CrossRef] [PubMed] [Google Scholar]
- Is there a role of faecal microbiota transplantation in reducing antibiotic resistance burden in gut? A systematic review and Meta-analysis. Ann Med. 2021;53:662-81.
- [CrossRef] [PubMed] [Google Scholar]
- Antibacterial efficacy and mechanisms of action of low power atmospheric pressure cold plasma: Membrane permeability, biofilm penetration and antimicrobial sensitization. J Appl Microbiol. 2018;125:398-408.
- [CrossRef] [PubMed] [Google Scholar]
- Photodynamic antimicrobial chemotherapy: Advancements in porphyrin-based photosensitize development. Front Chem. 2021;9:635344.
- [CrossRef] [PubMed] [Google Scholar]
- Metallic nanoparticles and their medicinal potential. Part II: aluminosilicates, nanobiomagnets, quantum dots and cochleates. Ther Deliv. 2013;4:1179-96.
- [CrossRef] [PubMed] [Google Scholar]
- Sonodynamic antimicrobial chemotherapy: First steps towards a sound approach for microbe inactivation. J Photochem Photobiol B. 2015;150:44-9.
- [CrossRef] [PubMed] [Google Scholar]
- The role of vaccines in combatting antimicrobial resistance. Nat Rev Microbiol. 2021;19:287-302.
- [CrossRef] [PubMed] [Google Scholar]
- India's National Action Plan on antimicrobial resistance: A critical perspective. J Glob Antimicrob Resist. 2021;27:236-8.
- [CrossRef] [PubMed] [Google Scholar]
- Challenges in implementing antimicrobial stewardship programmes at secondary level hospitals in India: An exploratory study. Front Public Health. 2020;8:493904.
- [CrossRef] [PubMed] [Google Scholar]
- Hospital-based antimicrobial stewardship, India. Bull World Health Organ. 2023;101:20-27A.
- [CrossRef] [PubMed] [Google Scholar]






