Translate this page into:
Six sigma matrix and Quality Goal Index ratio in improving the quality of analytical phase in a clinical laboratory
*Corresponding author: Sapna Vyakaranam, Department of Biochemistry, Mediciti Institute of Medical Sciences, Hyderabad, Telangana, India. vyakaranamsapna@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Vyakaranam S, Nori SN, Rehan AR, Pothula Y, Yasam L, Agiwal V. Six sigma matrix and Quality Goal Index ratio in improving the quality of analytical phase in a clinical laboratory. J Lab Physicians. 2024;16:515-22. doi: 10.25259/JLP_56_2024
Abstract
Objectives:
The objective of the study is to assess the performance of individual biochemical parameters on a sigma scale and also to do root cause analysis and take corrective actions for the parameters with poor performance to improve the quality of our clinical laboratory.
Materials and Methods:
This is a retrospective and prospective study done in the central laboratory of a tertiary care hospital from January 2023 to September 2023. The daily internal quality control (IQC) data and monthly external quality assessment service data for 10 biochemical parameters from January 2022 to December 2022 were collected retrospectively and from April 2023 to September 2023 prospectively. Parameters with poor sigma performance and root cause analysis were done, and corrective actions were taken. Data were collected prospectively for the next 4 months (April 2023–September 2023), and sigma was calculated.
Statistical analysis:
Data were input into Microsoft Excel and analyzed using Stata version 14.
Results:
Out of the ten, 7 parameters at level 1 and five at level 2 IQC showed sigma values between 3 and 6, whereas 2 parameters showed poor performance at both the quality control (QC) levels with sigma metrics values <3. With quality goal index and root cause analysis, the source of error was detected and corrected.
Conclusions:
Sigma metric analysis is a tool to determine the performance of QC design. This gives the laboratory a select the right QC strategy. This will help to save time, effort, unnecessary runs, calibration, and reagent waste, which affect the outcome of turnaround time.
Keywords
Sigma metrics
Imprecision
Inaccuracy
Quality goal index
Root cause analysis
INTRODUCTION
The clinical laboratory is the core of the healthcare system. Providing an accurate report is quite challenging as the diagnosis, treatment, and prognosis of the disease by a physician are in accordance with the report.[1] Here comes the importance of a quality management system (QMS) for releasing accurate reports. There are recommended guidelines provided by ISO15189 to assess and monitor the QMS.[2]
Quality control (QC) is one of the components of QMS, which monitors and evaluates the analysis phase of the clinical laboratory. QC is the statistical analysis of internal QC (IQC) and external quality assessment service (EQUAS) programs.[3] IQC is evaluated daily on Levey–Jenning (LJ) charts using west guard rules.[4] EQUAS analyses unknown concentrations of controls monthly, provided by an external agency. EQUAS is interpreted by Z SCORE or standard deviation index.[5] A Z-score is a calculated value that indicates how many standard deviations a control result has shifted from the mean value that is expected for that material. IQC checks the precision, and EQUAS checks the accuracy of the parameter to the mean value.[6]
Neither IQC nor EQUAS can give the exact number of errors occurring in the analytical phase of the clinical laboratory.[7] Six Sigma, which integrates accurate evaluation and process improvement, has come to light.[8] Six Sigma metrics evaluate the errors in the QC system and quantify the performance as “defects per million.”[9] The power of six sigma is measured on the “Sigma Scale.” It typically runs from 0 to 6, but a process can exceed six sigma if variability is sufficiently low to decrease the defect rate.
These values indicate the chance of false test results by the clinical laboratory, a value <3 Indicates poor performance, while >3 is considered as good and >6 is world-class performance.[10] Sigma metrics are a calculation in which sigma is a matrix that quantifies the performance as “defects per million” and EQUAS data along with total allowable error (TEa). Mao et al., in their study, stated that “Sigma metrics is a self-assessment method in guiding clinical laboratories to make QC strategy and plan QC frequency”[11]. Previous studies were done to elicit the individual laboratory performances but very few studies have reviewed and corrected the parameters with poor sigma scale.[4]
An accurate report is needed for proper clinical diagnosis. This study aims to assess the performance of individual biochemistry parameters on the sigma scale. In addition, we will conduct a root cause analysis and implement corrective actions for parameters that perform poorly on the sigma scale to enhance the overall performance of our clinical laboratory.
MATERIALS AND METHODS
This is a retrospective-prospective study conducted in the central laboratory of a tertiary care hospital from January 2023 to September 2023. Retrospective data of daily IQC and monthly EQUAS from January 2022 to December 2022 were collected. Subsequently, daily IQC and monthly EQUAS data were collected prospectively from April 2023 to September 2023. The performance of the following ten biochemistry parameters was studied: Serum creatinine, total bilirubin, total protein, albumin, calcium, triglycerides, high-density lipoprotein cholesterol (HDL-C), aspartate transaminase (AST), alkaline phosphatase (ALP), and amylase.
Study procedure
The parameters were analyzed on the Beckman AU480 autoanalyzer. IQC material was obtained in lyophilized form from Bio-Rad. After the instrument’s daily maintenance, two levels of Bio-Rad IQC L1 and L2 were run. Both the levels of IQC data were plotted as LJ charts and were interpreted using Westgard rules. Only after the QC values were in range, patient samples were analyzed. Monthly EQUAS was done on lyophilized samples obtained from Christian Medical College, Vellore. EQUAS report was uploaded by the 20th of every month. An standard deviation index (SDI) value of ±2 was considered acceptable. For any unacceptable results parameters, corrective actions were taken.
Data collection and calculations
The IQC data for the 10 parameters serum creatinine, total bilirubin, total protein, albumin, calcium, triglycerides, HDL-C, AST, ALP, and amylase were collected retrospectively. The performance of these 10 biochemical parameters was done on a Sigma Scale by calculating the sigma metrics for each parameter.
Sigma metrics were calculated with the following formula:[12]
Where TEa is the total allowable error and Bias and coefficient of variation (CV) are the indicators of systematic and random errors, respectively.
TEa for each parameter was obtained from Clinical Laboratory Improvement Amendment proficiency testing criteria for acceptable analytical performance printed in the federal register.[13]
Bias is the systematic difference between the expected results obtained by the laboratory test method and the results obtained from an accepted reference method. The bias percentage for each parameter was calculated from the EQAS report using the formula.[14]
CV was calculated for the Bio-Rad IQC each month. CV is a measure of the variability of an assay and is expressed as a percentage.
The Sigma value was calculated for all the 10 parameters.
The standardized sigma values were categorized into six categories, i.e., world-class (σ ≥6), excellent (6-5), good (<5-4), marginal (<4-3), poor (<3-2), and unacceptable (<2).[10] For the parameters with sigma values 3, Quality goal index (QGI) was calculated. Based on the values of QGI, the reason for the poor sigma performance of the parameters was interpreted. A value of <0.8 indicates imprecision, 0.8–1.2 indicates imprecision and inaccuracy, and >1.2 indicates inaccuracy.[15,16] The formula used for QGI was:
With reference from QGI, root cause analysis (RCA) was performed, and corrective actions were taken using a cause-effect chart[17] (fishbone diagram), as represented in Figure 1. After corrective actions, the sigma values for the parameters were calculated from April 2023 to September 2023, that is, for 6 months.
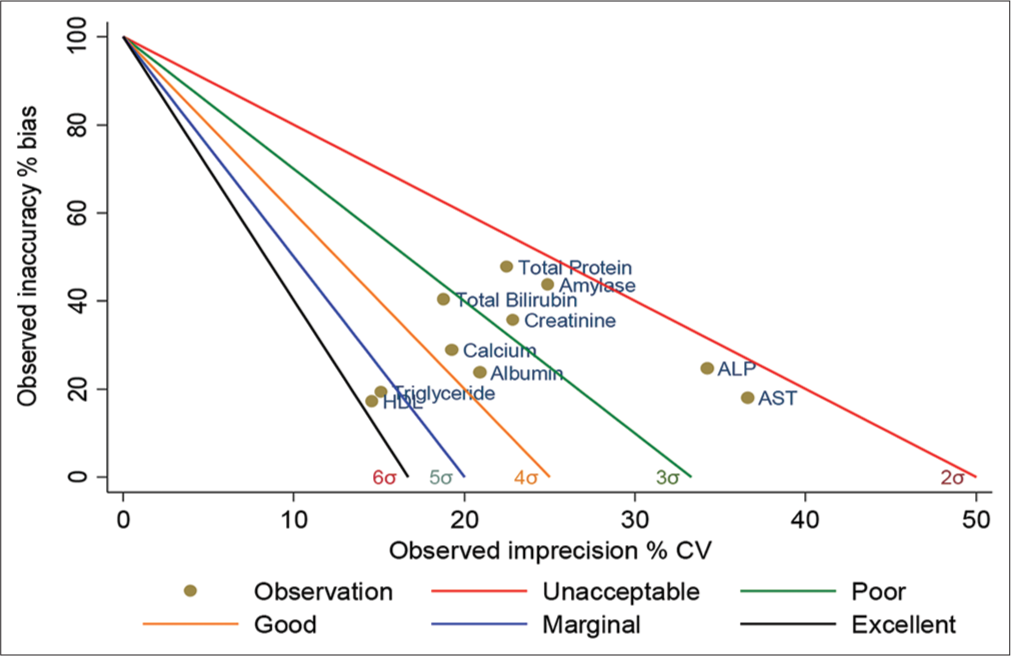
- Standardized QC sigma chart for 10 analytes (level-1). The slope of the five lines is the negative value of sigma. The colored circles represent the sigma value of the analytes. The X-axis is the percentage of CV normalized to TEa, and the Y-axis is the percentage of bias normalized to TEa. QC: Quality control, TEa: Total allowable error, CV: Coefficient of variation. HDL: High-density lipoprotein, AST: Aspartate transaminase, ALP: Alkaline phosphatase.
Statistical analysis
Data were input into Microsoft Excel and analyzed using Stata version 14. We determined the internal data quality for 10 biochemical parameters by computing the CV, total allowable error, average bias, and sigma metric values. In addition, we calculated the quality global index (QGI) ratio for both QC material levels, i.e., Level 1 and 2. Bias and CV were computed using EQUAS and IQC data, respectively. To assess the performance of the biochemical parameters, we utilized a Normalized Method Decision Chart. The normalized operating point was determined using the Normalized IPsec calculator and plotted using Stata version 14.
RESULTS
Tables 1 and 2 represent the monthly and average CV of levels 1 and 2 IQC from January 2022 to December 2022, respectively. It was observed that CV % of both levels of IQC for the parameters creatinine, total bilirubin, total protein, albumin, calcium, triglycerides, L1 of HDL, and L2 of amylase was <5%; both L1 and L2 of AST and amylase were <10%.
| Parameter | CV percentage of level 1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| January | February | March | April | May | June | July | August | September | October | November | December | Average | |
| Creatinine | 3.1 | 4.19 | 3.14 | 2.81 | 3.44 | 2.34 | 3.48 | 3.74 | 2.97 | 4.25 | 3.66 | 4.06 | 3.43 |
| Total bilirubin | 5.82 | 3.38 | 2.35 | 2.84 | 2.95 | 2.64 | 2.07 | 7.2 | 3.48 | 4.63 | 2.93 | 4.82 | 3.76 |
| Total protein | 3.98 | 2.75 | 2.17 | 1.55 | 2.16 | 2.03 | 2.55 | 1.85 | 2.91 | 1.18 | 1.39 | 2.42 | 2.25 |
| Albumin | 2.07 | 1.92 | 2.98 | 2.59 | 2.6 | 1.7 | 1.3 | 2.51 | 1.59 | 2.15 | 1.94 | 1.7 | 2.09 |
| Calcium | 1.72 | 1.52 | 2.84 | 1.61 | 3.43 | 2.48 | 3.28 | 2.04 | 3.14 | 2.24 | 0.73 | 0.43 | 2.12 |
| Triglyceride | 3.75 | 1.98 | 5.69 | 3.92 | 2.92 | 4.74 | 2.58 | 10.72 | 1.75 | 1.11 | 3.7 | 2.49 | 3.78 |
| HDL | 4.21 | 8.95 | 2.79 | 2.47 | 2.59 | 3.11 | 3.09 | 3.16 | 4.77 | 4.09 | 5.85 | 7.37 | 4.37 |
| AST | 2.43 | 6.7 | 5.05 | 8.99 | 11.5 | 3.77 | 7.97 | 5.69 | 6 | 8.8 | 6.3 | 14.68 | 7.32 |
| ALP | 15.56 | 12.79 | 12.26 | 15.18 | 7.9 | 8.05 | 9.91 | 11.78 | 8.6 | 6.65 | 7.28 | 7.29 | 10.27 |
| Amylase | 9.8 | 4.53 | 7.21 | 7.86 | 10.61 | 6.64 | 7.83 | 3.36 | 8.67 | 6.16 | 7.86 | 9.11 | 7.47 |
HDL: High-density lipoprotein, AST: Aspartate transaminase, ALP: Alkaline phosphatase, CV: Coefficient of variation
| Parameter | CV percentage of level 2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| January | February | March | April | May | June | July | August | September | October | November | December | Average | |
| Creatinine | 1.57 | 2.23 | 1.48 | 1.48 | 2.13 | 1.57 | 3.02 | 2.61 | 3.14 | 4.12 | 2 | 3.16 | 2.38 |
| Total bilirubin | 5.54 | 3.48 | 3.3 | 4.38 | 4.42 | 3.37 | 6.76 | 5.16 | 2.9 | 5.62 | 6.22 | 1.42 | 4.38 |
| Total protein | 4.11 | 2 | 2.77 | 1.51 | 2.46 | 1.58 | 1.71 | 1.56 | 2.81 | 3.22 | 2.29 | 2.49 | 2.38 |
| Albumin | 1.61 | 2.08 | 2.82 | 2.59 | 2.71 | 1.86 | 1.48 | 2.35 | 1.67 | 2.87 | 2.82 | 2.02 | 2.24 |
| Calcium | 1.38 | 2.08 | 3.01 | 1.58 | 2.11 | 2.19 | 2.81 | 1.79 | 2.95 | 2.22 | 0.9 | 1.01 | 2 |
| Triglyceride | 4.04 | 2.22 | 5.18 | 3.25 | 3.26 | 4.69 | 2.65 | 3.13 | 4.59 | 4.33 | 3.11 | 3.72 | 3.68 |
| HDL | 5.39 | 6.14 | 2.54 | 1.65 | 10.1 | 6.18 | 3.48 | 8.14 | 11.7 | 3.3 | 10.98 | 8.91 | 6.55 |
| AST | 3.21 | 2.22 | 3.03 | 7.36 | 8.89 | 2.52 | 2.75 | 5.4 | 4.78 | 7.48 | 7.72 | 9.51 | 5.41 |
| ALP | 9.71 | 11.76 | 16.01 | 8.95 | 6.65 | 5.96 | 8.21 | 6.14 | 4.13 | 3.85 | 7.66 | 5.52 | 7.88 |
| Amylase | 3.12 | 2.26 | 4.24 | 5.53 | 5.68 | 3.48 | 3.28 | 2.65 | 3.79 | 2.33 | 7.11 | 4.45 | 3.99 |
HDL: High-density lipoprotein, AST: Aspartate transaminase, ALP: Alkaline phosphatase, CV: Coefficient of variation
Table 3 represents the Bias percentage of EQUAS for all the parameters. It was observed that all parameters had bias <10% except amylase, which was 13.5%.
| Parameter | Bias Percentage | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| January | February | March | April | May | June | July | August | September | October | November | December | Average | |
| Creatinine | 2.65 | 1.92 | 7.85 | 5.77 | 1.34 | 6.83 | 3.42 | 7.76 | 6.84 | 4.19 | 11.49 | 4.23 | 5.36 |
| Total bilirubin | 4.47 | 19.35 | 1.38 | 3.31 | 4.66 | 13.21 | 1.64 | 5.19 | 1.62 | 16 | 12.5 | 13.75 | 8.09 |
| Total protein | 25.48 | 4.93 | 5.1 | 0.73 | 2.93 | 1.12 | 3.49 | 2.77 | 0.98 | 4.96 | 3.21 | 1.61 | 4.78 |
| Albumin | 0.95 | 0.68 | 7.3 | 5.09 | 0.39 | 1.21 | 0.6 | 1.37 | 3.49 | 1.84 | 2.05 | 3.55 | 2.38 |
| Calcium | 3.48 | 2.7 | 2.12 | 0.43 | 3.99 | 7.01 | 2.34 | 0.99 | 2.9 | 1.54 | 3.08 | 7.48 | 3.17 |
| Triglyceride | 1.86 | 1.62 | 5.14 | 2.61 | 1.21 | 5.85 | 7.68 | 1.82 | 3.26 | 6.48 | 9.59 | 11.02 | 4.84 |
| HDL | 6.4 | 2.01 | 4.17 | 5.3 | 0.92 | 10.67 | 8.42 | 1.75 | 1.78 | 4.77 | 3.08 | 13.14 | 5.2 |
| AST | 0.25 | 1.5 | 0.55 | 3.56 | 2.63 | 4.5 | 18.32 | 0.59 | 6.2 | 1.34 | 0.81 | 2.89 | 3.6 |
| ALP | 25.44 | 18.68 | 17.33 | 0.37 | 4.28 | 0.51 | 2.15 | 7.94 | 3.39 | 0.92 | 4.87 | 3.42 | 7.44 |
| Amylase | 27.41 | 0.76 | 7.41 | 20.1 | 22.1 | 11.81 | 22.86 | 10.06 | 2.33 | 4.15 | 16.61 | 12.32 | 13.15 |
HDL: High-density lipoprotein, AST: Aspartate transaminase, ALP: Alkaline phosphatase
Table 4 represents Sigma METRICS for all the parameters. It was observed that sigma values for both levels of IQC for parameters albumin, calcium, triglycerides, HDL, L1 of total bilirubin, L2 of creatinine, AST, and amylase were >3. Whereas both the levels of total protein and ALP, L1 of creatinine, AST, and amylase, and L2 of total bilirubin were <3.
| Parameter | Tea (CLIA) | Average Bias | Level 1 | Level 2 | ||
|---|---|---|---|---|---|---|
| CV | Sigma | CV | Sigma | |||
| Creatinine | 15 | 5.36 | 3.43 | 2.81 | 2.38 | 4.05 |
| Total bilirubin | 20 | 8.09 | 3.76 | 3.17 | 4.38 | 2.72 |
| Total protein | 10 | 4.78 | 2.25 | 2.32 | 2.38 | 2.2 |
| Albumin | 10 | 2.38 | 2.09 | 3.65 | 2.24 | 3.4 |
| Calcium | 11 | 3.17 | 2.12 | 3.69 | 2 | 3.91 |
| Triglyceride | 25 | 4.84 | 3.78 | 5.32 | 3.68 | 5.48 |
| HDL | 30 | 5.2 | 4.37 | 5.67 | 6.55 | 3.79 |
| AST | 20 | 3.6 | 7.32 | 2.24 | 5.41 | 3.03 |
| ALP | 30 | 7.44 | 10.27 | 2.2 | 7.88 | 2.86 |
| Amylase | 30 | 13.15 | 7.47 | 2.26 | 3.99 | 4.22 |
TEa: Total allowable error, CV: Coefficient of variation, HDL: High-density lipoprotein, AST: Aspartate transaminase, ALP: Alkaline phosphatase, CLIA: Clinical laboratory improvement amendment
Figures 1 and 2 represent operational process specifications (OP) spec graphs. The slope of the five lines is the negative value of sigma. The colored circles represent the sigma value of the parameters; the X-axis is the percentage of CV normalized to TEa and shows imprecision, and the Y-axis is the percentage of bias normalized to TEa and shows inaccuracy.
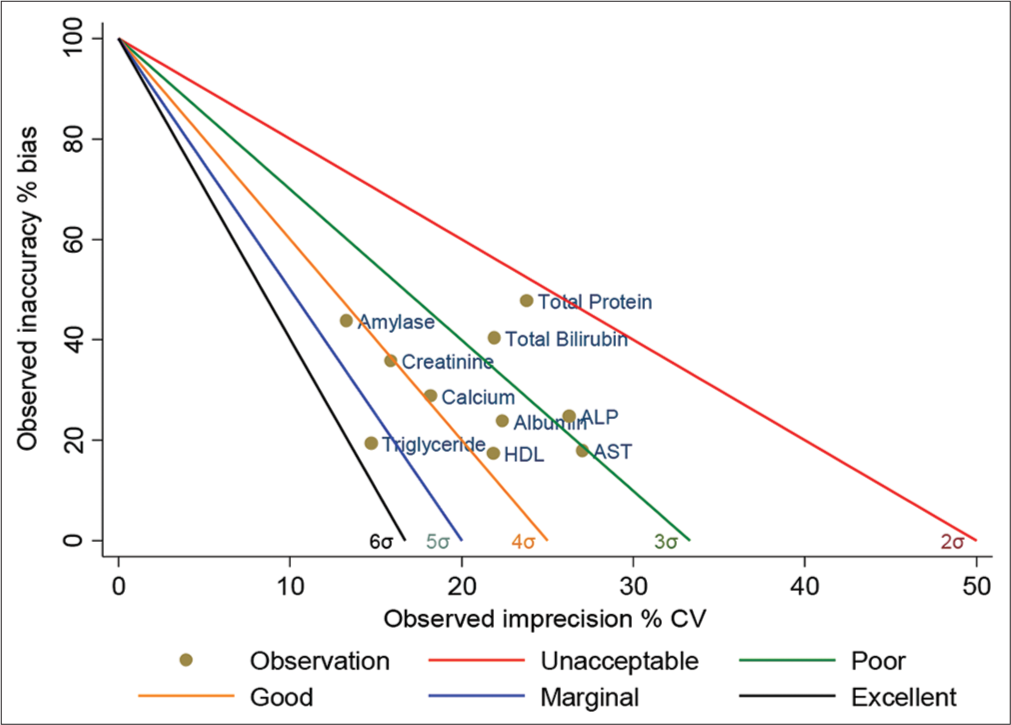
- Standardized QC sigma chart for 10 analytes (level-2). The slope of the five lines is the negative value of sigma. The colored circles represent the sigma value of the analytes. X-axis is the percentage of CV normalized to TEa, and the Y-axis is the percentage of Bias normalized to TEa. QC: Quality control, TEa: Total allowable error, CV: Coefficient of variation. HDL: High-density lipoprotein, AST: Aspartate transaminase, ALP: Alkaline phosphatase.
Table 5 represents QGI parameters whose level 1, level 2, or both sigma values were <3. It was observed that the cause for the poor sigma values for L1 and L2 of total protein and total bilirubin were inaccuracy. The poor sigma values for L1 and L2 of AST and ALP was imprecision.
| Parameter | Level 1 | Level 2 | ||||
|---|---|---|---|---|---|---|
| Sigma | QGI | Cause | Sigma | QGI | Cause | |
| Creatinine | 2.81 | 1.04 | Imprecision and inaccuracy | Not applicable | ||
| Total bilirubin | 3.17 | 1.43 | Inaccuracy | 2.72 | 1.23 | Inaccuracy |
| Total protein | 2.32 | 1.42 | Inaccuracy | 2.2 | 1.34 | Inaccuracy |
| AST | 2.24 | 0.33 | Imprecision | 3.03 | 0.44 | Imprecision |
| ALP | 2.2 | 0.56 | Imprecision | 2.86 | 0.68 | Imprecision |
| Amylase | 2.26 | 1.17 | Imprecision and inaccuracy | Not applicable | ||
AST: Aspartate transaminase, ALP: Alkaline phosphatase, QGI: Quality Goal Index
Table 6 represents RCA done as per the cause-effect chart and appropriate the corrective actions are taken. Figure 3 represents the cause effect chart (Fish bone analysis) for identifying cause and effect on parameters with poor sigma performance.
| S. No. | Parameter | Root cause analysis | Corrective action |
|---|---|---|---|
| 1. | AST, ALP, amylase | • Incubation chamber temperature | • Regular monitoring of the temperature was done • Stability of the reagent packs was verified |
| 2. | Total protein, total bilirubin, creatinine | • Reagent dispensing system • Sampling system |
• Major instrument maintenance was done |
| 3. | AST, ALP | • SD was narrowed | • 60 data points from daily IQC were collected laboratory, after removing the outliers, laboratory mean and SD were set up |
AST: Aspartate transaminase, ALP: Alkaline phosphatase, SD: Standard deviation, IQC: Internal quality control
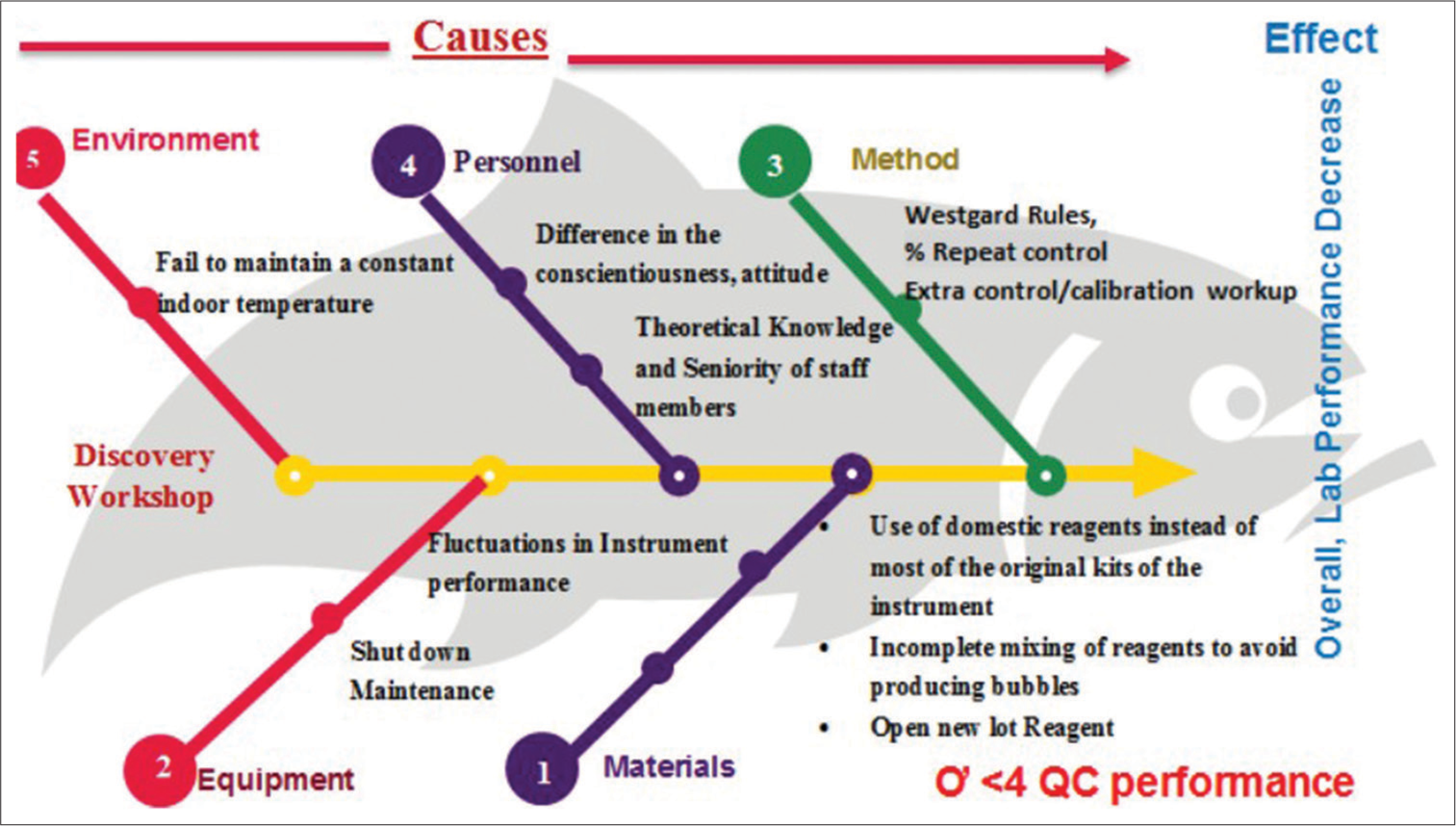
- Cause-effect chart (Fish-bone diagram) for the potential cause and effect for parameters with low sigma levels. QC: Quality Control
Tables 7 and 8 represent the CV of levels 1 and 2 IQC from April 2023 to September 2023, respectively. Table 9 represents the sigma values for the 6 parameters after corrective action. Sigma values for L1 of creatinine and amylase; L1 and 2 of total bilirubin, total protein, AST, and ALP were observed to be >3.
| Parameter | CV percentage of level 1 | ||||||
|---|---|---|---|---|---|---|---|
| April | August | July | June | May | Sept | Average | |
| Creatinine | 7.48 | 6.01 | 3.96 | 2.98 | 2.84 | 2.49 | 4.29 |
| Total bilirubin | 5.81 | 1.28 | 3.13 | 3.33 | 2.24 | 5.09 | 3.48 |
| Total protein | 3.43 | 3.55 | 2.68 | 2.02 | 1.41 | 2.43 | 2.59 |
| AST | 5.82 | 3.39 | 3.73 | 4.81 | 2.87 | 4.69 | 4.22 |
| ALP | 9.6 | 6.02 | 7.46 | 7.69 | 6.41 | 13.7 | 8.48 |
| Amylase | 8.79 | 5.94 | 11.98 | 6.71 | 5.44 | 7.67 | 7.76 |
CV: Coefficient of variation, AST: Aspartate transaminase, ALP: Alkaline phosphatase
| Parameter | CV percentage of level 2 | ||||||
|---|---|---|---|---|---|---|---|
| April | August | July | June | May | Sept | Average | |
| Creatinine | 3.67 | 2.11 | 3.65 | 4.07 | 2.3 | 3.66 | 3.24 |
| Total bilirubin | 1.3 | 1.69 | 4.08 | 2.44 | 2.07 | 4.15 | 2.62 |
| Total protein | 2.09 | 5.04 | 3.12 | 1.55 | 1.66 | 2.95 | 2.74 |
| AST | 2.92 | 1.04 | 4.16 | 2.92 | 1.99 | 3.51 | 2.76 |
| ALP | 9.61 | 4.49 | 6.58 | 7.6 | 5.14 | 14.97 | 8.07 |
| Amylase | 2.66 | 2.76 | 4.74 | 7.3 | 2.45 | 7.97 | 4.65 |
CV: Coefficient of variation, AST: Aspartate transaminase, ALP: Alkaline phosphatase
| Parameter | TEa (CLIA) | Average Bias | Level 1 | Level 2 | ||
|---|---|---|---|---|---|---|
| CV | Sigma | CV | Sigma | |||
| Creatinine | 15 | 3.17 | 3.71 | 3.19 | ||
| Total bilirubin | 20 | 3.04 | 2.82 | 6.01 | 2.32 | 7.31 |
| Total protein | 10 | 1.68 | 2.56 | 3.25 | 1.96 | 4.24 |
| AST | 20 | 2.05 | 4.22 | 4.25 | 2.67 | 6.72 |
| ALP | 30 | 1.8 | 8.35 | 3.38 | 8 | 3.53 |
| Amylase | 30 | 2.3 | 6.29 | 4.4 | ||
TEa: Total allowable error, CV: Coefficient of variation, AST: Aspartate transaminase, ALP: Alkaline phosphatase, CLIA: Clinical laboratory improvement amendment
DISCUSSION
In this era with the advancement of medical sciences, clinicians are depending on clinical laboratories for proper diagnosis and treatment. Automation has become an integral part of laboratories to meet the increasing workload and decrease TAT. The working of automation is checked by Good Laboratory Practice as per the National Accreditation Board for Testing and Calibration Laboratories guidelines. Most of the clinical laboratories follow IQC and EQUAS, monitor LJ charts, follow Westgard rules, and do corrective actions for outliers to maintain the quality of reports.[18] However by introducing a QMS tool, sigma metrics, the quality of the report at the analytical phase of testing can be improved.[11]
A sigma level of more than 3 Standard Deviations (SD) is always desirable.[16] The Sigma model pursues a Plan, Do, Check, Act cycle for QMS. The QMS is dominated by defining, measuring, analyzing, improving, and controlling, which are salient features of six sigma metrics.[19]
In the present study, we obtained values of six sigma for 10 biochemical parameters of both L1 and L2 levels of IQC. In our study, we observed L1 IQC of total bilirubin, level 2 of amylase, AST, and creatinine, and both levels of albumin, calcium, TG, and HDL-C were between 3 and 6 sigma values. It was also observed that both IQC levels of total protein and ALP failed to meet minimum sigma quality performance with a value <3.
Similar studies were done by Kumar and Mohan,[14] Verma et al.,[20] Pradhan et al.,[21] and Maheshwari et al.[6] Variations in sigma values between our study and others can be due to the difference in the methodology, traceability calibrators, instrument, QC material, and other pre-analytical and analytical conditions.[14,22]
In our study, QGI was calculated for parameters whose Sigma values were poor (ie <3) parameters like total protein, ALP, creatinine , AST, Amylase, total bilirubin. Creatinine and amylase had problems of imprecision, and in accuracy (QGI 0.8–1.2), AST and ALP were imprecision (QGI <0.8), and total protein was in accuracy (QGI >1.2).
The cause-effect chart (Fish-bone diagram) for RCA was carried out to determine the cause of low sigma values and Westgard rules were used for corrective actions.[17,23]
Incubation temperature fluctuations were found to be cause for enzymatic reagents such as AST and ALT, as in the study conducted by Goel et al.[23] For creatinine, sampling, and reagent dispense issues were resolved by major instrument maintenance. For total bilirubin and total protein, SD was narrowed using 60 points of IQC data and setting up lab mean and SD. This is similar to the study done by Pradhan et al.[21] Other factors that can affect the quality are working conditions in the laboratory, instrument proficiency, frequent calibration, training of the staff about quality management, and troubleshooting.[24] Proper designing and implementing of
QC strategies can be done by evaluating the sigma matrix. Any QC procedure in a clinical laboratory should work for a high probability of error detection and a low probability of false rejection.[14] Six sigma metrics help in providing a strategy and plan for QC frequency.[5] Guidelines proposed by Westgard for QC planning as per the Six Sigma matrix are given in Table 10.[17] Charuruks, in their study, stated the sigma scale can be applied as a universal benchmark for the comparative evaluation of performance between tests, methods, equipment, and laboratories.[25] The errors encountered in the clinical laboratory can be decreased by prioritizing the quality improvement plan through the evaluation of low sigma value analytes and monitoring of daily quality indicators.[26]
| Sigma metrics | Levels of QC | Rules | No. of runs |
|---|---|---|---|
| ≥6σ | 1 | >13.5s | Once |
| 5σ | 2 | 1-3s | Twice |
| 4σ | 2 | 1–3 s, R4s | Twice |
| 3σ | 1–3 s, R4s, 2 of 2–2 s, 2 of 3–2 s, 4–1 s and 12x RCA and method performance is required before releasing the result | ||
QC: Quality control, RCA: Root cause analysis
CONCLUSIONS
Our study states that sigma metrics are a reliable quality tool for assessing the analytical performance of a clinical laboratory. However, a few parameters had sigma values <3, with RCA and corrective actions performed based on personnel, equipment, materials, method, and environment, sigma values raised above 3. Sigma metric analysis is a tool to determine the performance of QC design. This gives the laboratory a select the right QC strategy. This will help to save time, effort, unnecessary runs, calibration, and reagent waste, which affect the outcome of turnaround time.
Acknowledgment
Research reported in this publication was conducted by scholars in the Fogarty International Centre of the National Institutes of Health training program under Award Number D43 TW 009078. We would like to thank laboratory technicians for their contribution in acquisition of data.
Authors contribution
SV: Concept, design, the definition of intellectual content, literature search, data analysis, manuscript preparation, manuscript editing; SN.S: Design, the definition of intellectual content, literature search, data analysis, , manuscript editing, and manuscript review; ARR: Literature search, data acquisition, data analysis, manuscript editing, and manuscript review; YP: Literature search, data acquisition, data analysis, manuscript editing .and manuscript review; LY: Design, literature search, data acquisition, data analysis, manuscript editing and manuscript review; VA: Literature search, data analysis, statistical analysis, manuscript editing and manuscript review.
Ethical approval
The research/study was approved by the Medicit Institute of Medical Sciences, with approval reference no.: EC/05/XI/2k24, dated 05th November 2024.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Why is the laboratory an afterthought for managed care organizations? Clin Chem. 1996;42:813-6.
- [CrossRef] [PubMed] [Google Scholar]
- ISO 15189:2012 Medical laboratories-requirements for quality and competence. Geneva: International Organization for Standardization; 2012.
- [Google Scholar]
- Beyond quality assurance: Committing to quality improvement. Lab Med. 1998;20:241-7.
- [CrossRef] [Google Scholar]
- Internal quality control: Planning and implementation strategies. Ann Clin Biochem. 2003;40:593-611.
- [CrossRef] [PubMed] [Google Scholar]
- Tietz textbook of clinical chemistry and molecular diagnostics [5th edn] Ann Clin Biochem Int J Lab Med. 2012;49:615.
- [CrossRef] [Google Scholar]
- Quality improvement in clinical biochemistry laboratory using six sigma metrics and quality goal index. J Med Sci Res. 2021;9:101-7.
- [CrossRef] [Google Scholar]
- Beyond accuracy: What data quality means to data consumers. J Manag Inf Syst. 1996;12:5-33.
- [CrossRef] [Google Scholar]
- Six sigma as a quality management tool: Evaluation of performance in laboratory medicine In: Quality management and six sigma. London: IntechOpen; 2010.
- [CrossRef] [Google Scholar]
- Application of sigma metrics analysis for the assessment and modification of quality control program in the clinical chemistry laboratory of a tertiary care hospital. Indian J Clin Biochem. 2017;32:106-9.
- [CrossRef] [PubMed] [Google Scholar]
- Sigma metrics in clinical chemistry laboratory-A guide to quality control. Al Ameen J Med Sci. 2015;8:281-7.
- [Google Scholar]
- Evaluating analytical quality in clinical biochemistry laboratory using six sigma. Biochem Med (Zagreb). 2018;28:20904.
- [CrossRef] [PubMed] [Google Scholar]
- Practical application of six sigma management in analytical biochemistry processes in clinical settings. J Clin Lab Anal. 2020;34:e23126.
- [CrossRef] [PubMed] [Google Scholar]
- Defining allowable total error limits in the clinical laboratory. Adv Clin Chem. 2024;118:205-223.
- [CrossRef] [PubMed] [Google Scholar]
- Sigma metrics as a tool for evaluating the performance of internal quality control in a clinical chemistry laboratory. J Lab Physicians. 2018;10:194-9.
- [CrossRef] [PubMed] [Google Scholar]
- Quality indicators in laboratory medicine: From theory to practice. Preliminary data from the IFCC Working Group Project “laboratory errors and patient safety”. Clin Chem Lab Med. 2011;49:835-44.
- [CrossRef] [PubMed] [Google Scholar]
- The quality of laboratory testing today: An assessment of sigma metrics for analytic quality using performance data from proficiency testing surveys and the CLIA criteria for acceptable performance. Am J Clin Pathol. 2006;125:343-54.
- [CrossRef] [Google Scholar]
- Improving quality and efficiency performance of analytical testing process using sigma metrics in emergency laboratory of King Fahd Armed Forces Hospital, Jeddah, Saudi Arabia. Int J Adv Res. 2023;11:583-606.
- [CrossRef] [Google Scholar]
- Quality control review: Implementing a scientifically based quality control system. Ann Clin Biochem. 2016;53:32-50.
- [CrossRef] [PubMed] [Google Scholar]
- Equivalent quality testing versus equivalent QC procedures. Lab Med. 2005;36:626-9.
- [CrossRef] [Google Scholar]
- Assessment of quality control system by sigma metrics and quality goal index ratio: A roadmap towards preparation for NABL. World J Methodol. 2018;8:44-50.
- [CrossRef] [PubMed] [Google Scholar]
- Six sigma metrics: An evolving indicator of quality assurance for clinical biochemistry. J Clin Diagn Res. 2022;16:BC14-8.
- [CrossRef] [Google Scholar]
- Six sigma in clinical biochemistry: It matters, measure it. Int J Clin Biochem Res. 2017;4:270-4.
- [Google Scholar]
- Analysis of performance of clinical biochemistry laboratory using sigma metrics and quality goal index. Pract Lab Med. 2020;23:e00195.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of sigma metrics and Westgard rule selection and implementation of internal quality control in clinical chemistry reference laboratory, Ethiopian Public Health Institute. Ind J Clin Biochem. 2022;37:285-93.
- [CrossRef] [PubMed] [Google Scholar]
- Sigma metrics across the total testing process. Clin Lab Med. 2016;37:91-117.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of the analytical performance of endocrine analytes using sigma metrics. J Clin Lab Anal. 2021;35:e23581.
- [CrossRef] [PubMed] [Google Scholar]






