Translate this page into:
A Comparative Analysis of Polymerase Chain Reaction and Direct Fluorescent Antibody Test for Diagnosis of Genital Herpes
Address for correspondence: Dr. Preena Bhalla, E-mail: preenabhalla@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
To compare laboratory tests that can simultaneously detect and type herpes simplex virus (HSV) directly from the genital ulcer specimens in clinically suspected cases of genital herpes.
Materials and Methods:
A study was conducted over 10 months and 44 adult male and female patients clinically suspected with genital herpes were recruited. Genital ulcer swab specimens were subjected to glycoprotein-G gene-based conventional polymerase chain reaction (PCR) and commercially available direct fluorescent antibody (DFA) test and the results were compared.
Results:
PCR for HSV was positive in 82% (36/44) cases. DFA was positive in 68.2% (30/44) cases. There was 100% agreement between HSV types detected by DFA and PCR. The strength of agreement between the results was better in primary genital herpes than recurrent cases.
Conclusion:
PCR was found to be better in the detection of HSV in recurrent genital herpes patients. It is a better modality, especially when genital herpes clinically presents with ulcerative or crusted lesions, and is also a cheaper alternative as compared to DFA.
Keywords
Genital herpes
herpes simplex virus polymerase chain reaction
simultaneous detection and typing of herpes simplex virus
INTRODUCTION
Herpes simplex viruses (HSVs) exist as two major serotypes, HSV-1 and HSV-2 both of which can cause genital herpes.[1] The type of HSV infection affects prognosis and subsequent counseling; thus, type-specific testing to distinguish HSV-1 from HSV-2 is recommended.[234567] The aim of this study was to compare the laboratory tests which were able to simultaneously detect and type HSV directly from the genital ulcer specimens. In this study, we compared glycoprotein-G (gG) gene-based conventional polymerase chain reaction (PCR) with commercially available monoclonal antibody-based direct fluorescent antibody (DFA) test in a tertiary care hospital in New Delhi, India.
MATERIALS AND METHODS
With the institutional ethics committee approval, a study was conducted from July 2012 to January 2013 in which 44 adult male and female patients who were clinically suspected to have genital herpes were included. Patients who have received treatment for genital herpes in preceding 4 weeks were excluded from the study. The recruited patients were grouped on the basis of clinical presentation into five different categories – primary genital herpes and recurrent genital herpes on the basis of the time of presentation; and genital vesicles, ulcers, and crusted erosions on the basis of the type of genital lesion at the time of presentation. We did not include any healthy or other nonherpes ulcer control.
Clinical specimens
Genital ulcer swab specimens were separately collected for PCR and DFA with sterile dacron swabs which were immediately placed in viral transport medium (HiMedia, India) and transported to the laboratory in an ice box immediately and placed in a deep freezer at −70°C till further processing.
Preparation of genomic DNA
DNA extraction was performed using QiaAmp DNA mini kit (Qiagen, USA) blood or body fluids spin protocol and the extract was stored at −20°C in aliquots till used for amplification. HSV primers: Type-specific gG gene-based HSV primers were used as follows: HSV-1 (forward primer) 5'-CCCCCATGCCAAGTATTGGA-3', HSV-2 (forward primer) 5'-AGCTCCCGCTAAGGACATG-3', HSV-1 and 2 (reverse primer) 5'-AGACATACGTAACGCACGCT-3'.
Polymerase chain reaction condition
PCR reaction contained 25 μl of PCR master mix, 2 μl of forward and reverse HSV primers each (10 pm), 8 μl of DNA extract, and 7.5 μl of deionized nuclease free water to obtain a final volume of 50 μl. PCR amplification was carried out in GeneAmp PCR – 9700 thermocycler (Applied Biosystems, USA). The thermal profile was initial denaturation at 94°C for 3 min, followed by denaturation at 94°C for 45 s, annealing and extension at 72°C for 1 min (35 cycles), and final extension at 72°C for 5 min. PCR products were electrophoresed and visualized on 2% agarose. Amplicons were identified and differentiated as follows: HSV-1 gG PCR amplicon size – 487 bp and HSV-2 gG PCR amplicon size – 214 bp.
Direct fluorescent antibody test
It was performed using “Pathfinder HSV 1 and 2” (Bio-Rad Laboratories, USA) along with positive and negative control slides. Interpretation of the test was performed as follows: (1) Positive: Specimen containing at least one epithelial cell displaying specific apple green fluorescence in HSV-1 and/or HSV-2 well (s) and compared with positive control slides. (2) Negative: Specimen containing minimum of 10 epithelial cells in both HSV-1 and HSV-2 wells stained red due to the counterstain. The results of PCR and DFA were compared in all the above-mentioned categories based on clinical presentation of the disease.
Statistical analysis
The significance of difference between the two tests was calculated by McNemar test using Statistical Package for Social Science software (SPSS version 20.0, IBM Corp., Armonk, NY) (P < 0.05 was considered statistically significant). The measure of agreement between the two laboratory tests was determined by Kappa index, where value of 1.0 indicates complete agreement.
RESULTS
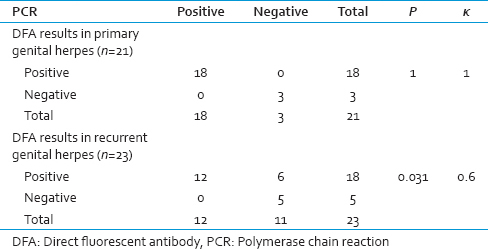
A total of 44 patients were recruited during the study of which 21 (48%) presented with primary genital herpes and 23 (52%) with recurrent genital herpes. Of the total patients, 31 (70%) presented with ulcerative genital lesions, 4 (9%) with vesicular lesions, and the remaining 9 (21%) with crusted erosions. PCR was positive in a total of 36 (82%) specimens of which 18 (85.71%) cases were of primary and 18 (78.26%) cases of recurrent genital herpes (P = 0.701). PCR was positive in all the cases of vesicular lesions, 81% cases of ulcerative lesions, and 78% cases with crusted erosions. DFA was also positive in all the cases of vesicular lesions, but in 71% cases with ulcerative lesions and in 44% cases with crusted erosions (P > 0.05) [Table 1].

When we compared our PCR results with DFA test, we found that there was a significant difference in results obtained by these tests in recurrent genital herpes cases (P = 0.031) but not in primary genital herpes (P = 1). The strength of agreement between the results of PCR and DFA was found to be better in primary genital herpes (κ =1) than recurrent cases (κ =0.6). There was 100% agreement in DFA and PCR results in vesicular lesions (κ =1). Similarly, the strength of agreement between the results was better in vesicular than ulcerative than crusted erosions [Tables 2 and 3].

DISCUSSION
In our study, PCR for HSV was positive in 82% (36/44) cases. Another study also showed that in 87.8% cases, HSV was demonstrated by real-time PCR.[8] However, the positivity rate depends on many variables including clinical diagnosis, clinical presentation of the disease, collection of the specimens, and transport to name a few. We found that there was no significant difference in the positive cases detected by PCR in either primary or recurrent genital herpes cases (P = 0.701). This finding is similar to the observation by Ramaswami et al.[9] We found that there was a significant difference in detection of positive cases by DFA and PCR in recurrent genital herpes (P = 0.031) but not in primary genital herpes (P = 1). There was 100% concordance in DFA and PCR results in vesicular lesions. We observed 100% agreement between HSV types detected by DFA and PCR. PCR was found to be better in diagnosis of the recurrent genital herpes patients and in healing lesions. Our findings are similar to the observation by Fang et al., who also used a similar gG gene-based PCR assay.[10] We also observed that although DFA test was easy to perform and required <1 h; it is costly and difficult to interpret. It also requires a fluorescent microscope which may not be available everywhere. However, PCR takes longer time and a little cumbersome to perform is easy to interpret and cheaper as compared to DFA test.
CONCLUSION
PCR was found to be better in the detection of HSV in recurrent genital herpes patients; however, in primary genital herpes cases, both DFA and PCR are comparable to each other. Similarly, both these modalities are equally good in diagnosis of genital herpes when it presents clinically with vesicular lesions, but PCR is a better option when genital herpes clinically presents with ulcerative or crusted lesions. Moreover, PCR is a cheaper alternative as compared to DFA.
Limitations
We neither performed HSV culture nor have the clinical outcome of the patients to be considered as gold standard for comparison. Inclusion of either of it would have enabled us to calculate sensitivity and specificity of both the tests for assessing the routine applicability of these tests.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull World Health Organ. 2008;86:805-12.
- [Google Scholar]
- Diagnostics for herpes simplex virus: Is PCR the new gold standard? Mol Diagn Ther. 2006;10:17-28.
- [Google Scholar]
- Characterization of herpes simplex virus clinical isolate Y3369 as a glycoprotein G variant and its bearing on virus typing. Virol J. 2011;8:290.
- [Google Scholar]
- Recurrence rates in genital herpes after symptomatic first-episode infection. Ann Intern Med. 1994;121:847-54.
- [Google Scholar]
- Recurrences after oral and genital herpes simplex virus infection. Influence of site of infection and viral type. N Engl J Med. 1987;316:1444-9.
- [Google Scholar]
- Clinical reactivation of genital herpes simplex virus infection decreases in frequency over time. Ann Intern Med. 1999;131:14-20.
- [Google Scholar]
- Herpes simplex virus: The importance of asymptomatic shedding. J Antimicrob Chemother. 2000;45:1-8.
- [Google Scholar]
- Detection and direct typing of herpes simplex virus in perianal ulcers of patients with AIDS by PCR. J Clin Microbiol. 1998;36:848-9.
- [Google Scholar]
- Diagnosis of genital herpes by real time PCR in routine clinical practice. Sex Transm Infect. 2004;80:406-10.
- [Google Scholar]
- Rapid detection of glycoprotein G gene for the diagnosis and typing of herpes simplex virus infection in genital herpes. Sex Transm Infect. 1999;75:396-7.
- [Google Scholar]





