Translate this page into:
A comparative evaluation of lithium estimation for samples collected in different tubes and its stability on storage
Address for correspondence: Dr. Arnab Pal, Department of Biochemistry, Postgraduate Institute of Medical Education and Research, Chandigarh - 160 012, India. E-mail: pal.arnab@pgimer.edu.in
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
PURPOSE:
Lithium (Li) is a well-established drug for the treatment of bipolar affective disorders. Li as a drug is known to possess a narrow therapeutic index. Thus, regular monitoring of blood Li in patients receiving Li therapy is essential. Plain tubes with clot activator are known to interfere with Li estimation. The current study was planned to compare Li estimation in sera from vacutainers with clot activator, and plasma from sodium heparinized vacutainers with that of Li estimation in sera from glass vials. The time-dependant stability of Li estimation on storage at 2°C–8°C for 48 h in these three set of tubes was also evaluated.
MATERIALS AND METHODS:
Blood from the patients on Li therapy (n = 100) was collected in 3 different collection tubes: plain vacutainer with clot activator (S), Sodium heparinized vacutainer (P) and Glass vial (G) and was analyzed by ion selective electrode (ISE) analyzer for Li levels. Secondary aliquots were also taken from each type of collection tube and stored at 2°C–8°C. Time-dependant stability of Li estimation was checked at 12 h, 24 h, and 48 h. ANOVA followed by Tukey's posttest was performed to calculate statistical significance taking glass vial as reference collection tube. Bland–Altman plots were plotted to compare between three collection tubes at baseline. Stability on storage was defined when >95% of the samples were within allowable error limit for that time point taking baseline levels as reference.
RESULTS:
A mean bias of 0.18 mmol/L and mean percentage bias of 19.9% in Li levels was observed between serum from (S) than serum from (G). This difference was found to be statistically significant. However, statistically nonsignificant mean bias of 0.02 mmol/L and mean percentage bias of 3.34% in Li levels was observed between plasma from (P) and serum from (G). Time-dependant stability was observed more in glass vials as compared to vacutainers with clot activator or sodium heparin.
CONCLUSION:
Serum from glass vial should be the preferred method for blood collection to determine Li levels.
Keywords
Bipolar disorder
ion selective electrode analyser
lithium
Introduction
Normally, lithium (Li) is present at a very low concentration in body fluids (<0.2 mmol/L). However, it has long been effectively used for prevention and treatment of bipolar affective disorders since the discovery of its role in bipolar disorders by Cade in 1950.[1] It successfully improves both manic and depressive episodes in 70%–80% of bipolar disorder patients.[2]
Serum/Plasma Li monitoring is, however essential owing to its narrow therapeutic index which lies between 0.6 mmol/L and 1.5 mmol/L below which no effect is seen, above which symptoms of Li intoxication appear.[2] The symptoms range from mild poisoning (1.5-2.0 mmol/L) with nausea, vomiting, diarrhea, and lethargy to severe (>2.5 mmol/L) with myocarditis, interstitial nephritis, coma, and death. Serum concentrations above 3–4 mmol/L generally require hemodialysis.[23]
Serum Li is estimated since many years, but plasma Li estimation has grossly been overlooked. The serum has been the specimen of choice for many years and is estimated most commonly by ion selective electrode (ISE), but plasma may be an equally valid or rather a preferable specimen for Li estimation. There are certain inherent drawbacks associated with serum samples like higher turnaround time because of the time taken for clot formation, and the risk of interference by fibrin clot on automated analyzers where there is no clot detection ability.[4] Moreover, there are reports in the literature that have shown a positive interference by silicone clot activator used in Vacutainers™ where they interact with ISE membrane leading to increased voltage signal thus, falsely elevating Li levels.[5] This can lead to false alarm and thus, mismanagement in the patients undergoing Li therapy and monitoring. The plasma may thus be considered as an alternative specimen for Li estimation. However, using ethylenediaminetetraacetic acid/oxalate as anticoagulant may result in false low levels of Li owing to complex formation. Thus, sodium heparin may be considered as the anticoagulant of choice for obtaining plasma for Li determination.
There are very few studies available where specimen type for Li estimation has been evaluated. To the best of our knowledge, no study is available on evaluation of stability on storage for Li estimation in different specimen types. In the current study, Li estimation in sera from vacutainers with clot activator and plasma from sodium heparinized vacutainers was compared with that of Li estimation in sera from glass vials. The time-dependant stability of Li estimation upon storage at 2°C–8°C at different time points over a period of 48 h in these three set of tubes was evaluated. Validation of published literature on interference by clot activators was also done.
Materials and Methods
Specimen selection, collection and handling
The present study was approved by the Institute's Ethics Committee, Postgraduate Institute of Medical education and Research, Chandigarh. A total of 100 patients attending the psychiatry outpatient department/Li clinic, emergency or admitted in the psychiatry ward and undergoing Li therapy or those who were advised for therapeutic monitoring, were enrolled for the study. Informed consent was obtained from the patients before the collection of venous blood.
Blood specimens were collected in the following types of vials:
-
G: 2.5-mL in Glass vial, no additives for serum
-
S: 2.5-mL in Plain BD Vacutainer with clot activator, no anticoagulant for serum
-
P: 2.5-mL in sodium heparinized, BD Vacutainer for plasma.
A volume of 7.5 ml triplicate blood samples (S, G, and P) from a single venipuncture were obtained from the antecubital vein using 10 ml syringe after proper antiseptic measures. Blood was transferred sequentially to G, S, and P tubes for either serum or plasma. Tubes were filled to capacity as per manufacturer's instructions and mixed gently but thoroughly.
All specimens were transported and processed in the laboratory within 1 h. Similar treatment was given to respective triplicates during processing. Serum was allowed to clot for 20 min at room temperature (25°C). Primary specimens (S, G, and P) were simultaneously centrifuged at 3000xg for 10 min. Immediately following centrifugation, three cell-free aliquots (~200 μL) were separated from each primary tube and stored at 2°C–8°C. Primary specimens were then analyzed for Li on ISE analyzer in the same run. Secondary aliquots were taken and analyzed for Li levels after 12, 24, and 48 h to check the stability of the samples.
Instrumentation
Li levels were analyzed with direct ISE method on the Eschweiler Combiline™, Germany. The analyzer undergoes automatic calibration with manufacturer provided calibrator. Two levels of internal quality control (IQC) were run and IQC data were found to be acceptable before the samples were analyzed for Li.
Data analysis
Statistical significance between Li levels in three different collection tubes was determined by one-way ANOVA using Prismv5 software™ taking glass vial as reference collection tube. When the ANOVA analysis indicated statistically significant difference, Tukey's posttest was employed to establish which pair of conditions differed significantly. The value of P < 0.05 was considered to be statistically significant. Bland Altman plots were plotted to show agreement between glass vial and the other two collection tubes. Both mean bias and percentage bias were determined using Bland–Altman analysis.
For determining time-dependant stability of Li analysis, difference between Li levels at each time point and baseline reference was calculated as:
D (Li) = (Li) tn – (Li) t0,
Where, (Li) tn represents Li levels at any time point and n = 12 h, 24 h or 48 h and (Li) t0 represents Li levels at baseline time. As per the recommendation by the Clinical Laboratory Improvement Amendments, for acceptable analytical performance of Li estimation, difference/bias between two values of Li, as analyzed by different methods should be <0.3 mmol/L or <20%.[6] Keeping this in view, acceptable difference/allowable error limit at different time points was taken as <0.3 mmol/L of Li.
The percentage of D (Li) values within allowable error limit (%WAEL), i e., with a difference of <0.3 mmol/L between Li at time t and Li levels at baseline was then calculated by the formula given below as suggested by Van Vrancken et al.[7]
%WAEL = number of D (Li) within ± allowable error limit/total number of values × 100
Maximum acceptable stability was defined when >95% values at given time point were within an acceptable limit. The statistically significant difference between Li levels at a given time point (tn) taking baseline (t0) as reference was determined by one-way ANOVA followed by Tukey's posttest using the Prismv5 software™.
Results
Comparison between different collection tubes at baseline
Figures 1 and 2 shows the Bland–Altman graphs for Li levels in heparinized vacutainers (P) and plain vacutainers with clot activator (S) with reference to glass tubes (G), respectively. In these plots, mean ± 2 standard deviation (SD) of bias (the difference between Li levels at baseline) between Li levels of two set of tubes is plotted against average of Li levels in these tubes.

- Bland altman plot for comparing Lithium levels in sodium heparinised evacuated collection tube (P) as compared to glass vial (G). Difference between serum lithium in glass vial (G) and plasma lithium levels in sodium heparinised evacuated collection tube (P) is plotted with average of the serum Li values from both the tubes. Acceptable bias <0.3 mmol/L
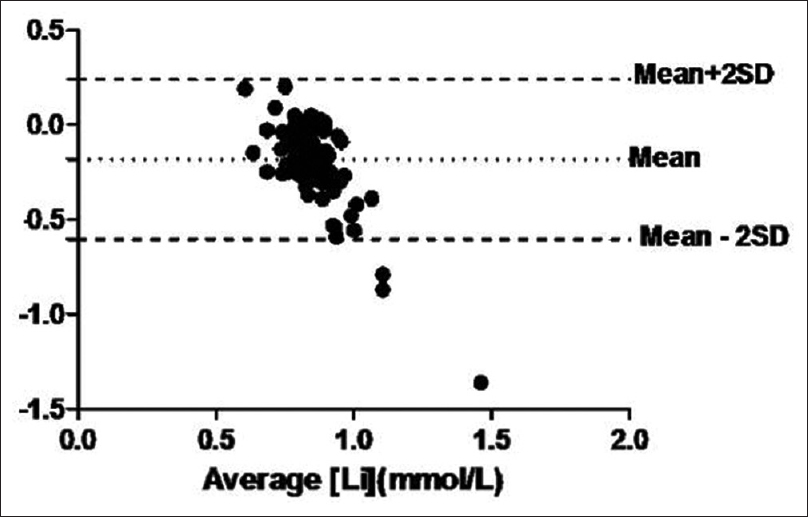
- Bland Altman plot for comparing Lithium levels in plain evacuated collection tube (S) with clot activator as compared to glass vial (G). Difference between serum lithium in glass vial (G) and Serum from plain evacuated collection tube with clot activator is plotted with average of the serum lithium values from both the tubes. Acceptable bias <0.3 mmol/L
As depicted in Figure 1, a mean bias of 0.02 mmol/L with an SD of 0.09 mmol/L is observed when Li levels in heparinized vacutainers (P) are compared with Li levels in glass vials (G). The bland-altman analysis in the above-mentioned set of tubes showed mean percentage bias of 0.18% with SD of 0.21%. No significant difference was observed between Li levels of P (0.73 ± 0.09 mmol/L.) and G (0.76 ± 0.10 mmol/L) set of tubes by one-way ANOVA and Tukey's posttest.
As shown in Figure 2, bland altman analysis between Li levels in plain vacutainer with clot activator (S) and glass vials (G) showed a higher mean bias of - 0.18 mmol/L. The percentage bias was found to be 19.9% ± 20.3%. Moreover, the difference in Li values between S (0.86 ± 0.29 mmol/L) and G (0.76 ± 0.10 mmol/L) was found to be statistically significant by ANOVA.
This clearly shows the significantly higher bias of Li estimation in clot activator plain vacutainers (S) than from heparinized vacutainers (P) relative to glass vials.
Time-dependant stability of lithium levels
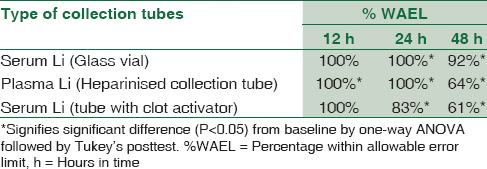
Table 1 shows the percentage of D (Li) values, i.e., (Li) at tn time - (Li) at t0 time, within allowable error limit (%WAEL), i.e., difference <0.3 mmol/L. Table also shows the statistical significant difference between Li levels at a given time point (tn) taking baseline (t0) as a reference. The serum Li levels in glass vials showed higher stability than plasma Li in heparinized tubes. Estimation of Li levels from sera in glass vials (G) showed higher stability wherein, 100% samples showed fall in Li levels <0.3 mmol/L (WAEL) at 12 h and 24 h, whereas as many as 92% were stable even at the end of 48 h. Similarly, the plasma in heparinized tube (P) also showed 100% samples WAEL till 24 h. However, only 64% remained stable at the end of 48 h. Moreover, statistically significant difference was observed at the end of 12 h from baseline in samples stored in heparinized tubes (P) as compared to at 24 h in that of glass vials (G). Li estimation in samples collected in plain vacutainers with clot activator (S) showed a poor stability with %WAEL falling to 83% at the end of 24 h and further to 61% at the end of 48 h.
Discussion
This is the first study where serum and plasma Li levels have been evaluated for their comparability and for their stability with time. It has been found in the present study, that serum from glass vial and plasma from sodium heparinized collection tubes yield comparable results at baseline. However, plain tubes with clot activator shows higher bias and statistically significant higher levels of Li than glass vials. Thus, the present study further validates the previous study by Sampson et al.,1997.[5] In their study, it was shown that silicon clot activator and surfactants used in plain vacutainers interact with the membrane of Li ISE. This interaction results in false elevation of Li values on the ISE analyzers.
When stored at 2°C–8°C, serum from glass vials confers higher stability with more samples within allowable error limit as compared to plasma from sodium heparinized collection tubes that start reducing significantly after 12 h and 24 h, respectively. Plain tubes with clot activator showed poor time-dependant stability relative to the other two collection tubes. Reports are available in the literature wherein stability of various analytes in serum and plasma have been compared and it was concluded that stability of analytes in serum is higher than plasma consistent with the present study.[8] However, they did not analyze Li as Li heparin vials were used. Hemo-concentration or interaction with Li-heparin was attributed for the observed changes in their study. In another study, Dorothea et al., 2013 have shown that serum refrigeration prolongs analyte stability; however, plasma offers stability challenges owing to in vitro fibrin formation, increased platelet activation on refrigeration and the presence of particulate in plasma resulting in preanalytical errors with time.[9]
The decrease in levels of Li in serum with time from clot activator plain collection tubes (S) is consistent with the previous report by Sampson et al.,[5] where they have shown fall in Li levels in serum due to a decrease in interference by silicone with time. The decrease of Li in glass vials and heparinized plasma with time could also be explained by the above-mentioned study where they have observed inverse relation of pH with Li ISE, i.e., as the pH falls Li levels increase with ISE. Since pH of serum/plasma increases with storage, it might result in fall in Li levels. Another study[10] has advocated Li estimation to be performed within 6 h as its levels falls after that and they have also attributed it to change in pH affecting the cationic assays. These could be the possible explanation for the fall in Li levels with time in the present study.
Conclusion
The current practice of estimating serum Li levels in plain tubes with clot activator should be abandoned. Collection of the specimen in these tubes for Li gives spuriously higher values and should be used with caution. Plasma from sodium heparinized vacutainer can, however, be used with reasonably reliable results with ease of processing. However, serum from glass vials with no additive gives the most reliable results and shows long term stability relative to plasma. Where ever possible, for Li estimation, glass vial should be collection tube of choice. However, if only vacutainers are available, sodium heparinized vacutainers should be preferred over plain vacutainers with clot activator for Li estimation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Clinical manifestations and management of acute lithium intoxication. Am J Med. 1994;97:383-9.
- [Google Scholar]
- Evaluation of three different specimen types (serum, plasma lithium heparin and serum gel separator) for analysis of certain analytes: Clinical significance of differences in results and efficiency in use. Clin Chem Lab Med. 2006;44:662-8.
- [Google Scholar]
- Positive interference in lithium determinations from clot activator in collection container. Clin Chem. 1997;43:675-9.
- [Google Scholar]
- Available from: http://www.westgard.com›CLIA and Quality›QualityRequirements
- Time-dependant stability of 22 analytes in lithium-plasma specimens stored at refrigerator temperature for upto 4 days. Lab Med. 2012;43:268-75.
- [Google Scholar]
- Stability studies of twenty-four analytes in human plasma and serum. Clin Chem. 2002;48:2242-7.
- [Google Scholar]
- Evaluation of refrigerated stability of 15 analytes in lithium heparin gel primary tubes. Lab Med. 2013;44:E45.
- [Google Scholar]





