Translate this page into:
Comparative Evaluation of Serum Lithium Estimation Using Plain Glass Vial and Serum Clot Activator Vacutainer by Reflectance Photometry
Address for correspondence: Sibasish Sahoo, MBBS, MD (Biochemistry), Department of Biochemistry, All India Institute of Medical Sciences, Kalyani, West Bengal 741245, India (e-mail: drsibasish18@gmail.com).
-
Received: ,
Accepted: ,
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction
The collection of blood samples in different vacutainers can affect the result of serum lithium estimation due to the presence of distinct additives in the blood collection vacutainer for enhancing the clot formation process. Due to the low therapeutic index and threat of toxicity of lithium, it is imperative to correctly report the test result. Thus, it has become a challenge for the laboratory physician to estimate lithium in any clinical laboratory setup.
Materials and Methods
Sample of 100 patients were collected and paired into clot activator vacutainers and plain glass vials. After centrifugation, samples from the paired collection tubes were processed immediately for serum lithium estimation by VITROS 4600 analyzer working on the principle of reflectance photometry. Both the paired tubes were stored at 2 to 8°C and were further analyzed, at 24 and 48 hours, respectively, from the time of their collection. The statistical analysis was done in IBM SPSS software version 23.
Results
There was a statistically significant differences between the mean of lithium values when processed within 1st hour of collection, obtained from clot activator vacutainers in comparison to glass vials. However, within tube comparison, there was no statistical difference in the lithium values estimated at 1st hour, 24 hours, and 48 hours of collection.
Conclusion
In this study, lithium values measured by clot-activated vacutainers are found to be lower as compared with values measured through glass vials.
Keywords
lithium
blood collection vacutainer
reflectance photometry
Introduction
In clinical practice, the prescription of oral lithium (Li) started with the treatment of gout[1] but subsequently, it has proven to be a potent drug in the treatment of bipolar mood disorder.[2] Due to its narrow therapeutic window that lies between 0.6 and 1.5 mmol/L, the therapeutic drug monitoring (TDM) of Li has to be measured at designated intervals to counteract the development of adverse effects of Li therapy. So, to maintain a constant concentration in a patient's bloodsera, the periodical laboratory assessment of Li cannot be overlooked.[3] The serum Li concentration should be monitored at an interval of every 1 to 3 months[4] in the patients undergoing Li therapy to avoid the serious toxicity effects of high doses such as tremors, renal failure, coma, or even death.
The currently recognized standard draw time for estimation of Li serum concentrations is at least 10 to 12 hours after the administration of the evening dose on a twice-daily dosing regimen.[5] Both serum and plasma are being used as sample specimens in laboratories for the determination of Li levels. For accurate monitoring of Li blood concentrations and complete evaluation of the method used, it requires proper validation of Li estimation with proper consideration of all preanalytical variables. Analytical factors such as specificity, selectivity, linearity, and total imprecision must be taken into account for good clinical laboratory practices.[6]
There are already published data that proclaim a positive interference by silica particles that is present as an added additive in the silicon coated vacutainer by its manufacturer to stabilize clot formation and reduce adherence of red cells to the inner tube wall in clot activator (red capped) vacutainers, where the silicone interacts with ions selective electrode (ISE) membrane leading to increased voltage signal thus, falsely elevating Li levels.[7] Incorrect overestimations lead to improper management of patients undergoing Li therapy and monitoring.
Several studies have estimated blood Li using different optical techniques such as the ISE method, fluorimetry, and spectrophotometry. Presumably, there is no study available on the evaluation of Li concentration and its stability on storage for Li estimation in different collection vacutainers by using dry slides reagent cartridge on the VITROS 4600 analyzer based on the reflectance photometry principle. In this line of work, the Li estimation from the bloodsera collected clot activator vacutainer was compared with that of Li values collected from the same patient bloodsera in a glass vial.
Materials and Methods
During the study period of 6 months (July 2022 to December 2022), a total of 100 patients who attended the Mood Clinic of Psychiatry Department, All India Institute of Medical Sciences, Kalyani, fulfilling the inclusion and exclusion criteria were included in this study. Blood samples were collected and analyzed from all enrolled the patients who were advised for therapeutic monitoring of serum Li to the biochemistry clinical laboratory, so no exclusion of patients was made. A volume of 5 mL of blood was drawn from the antecubital vein using a 5 mL syringe following all antiseptic precaution measures. Immediately after collection, 2.5 mL of blood was equally transferred into the plain glass vials (without any additives) and the red capped clot activator vacutainers (containing silica particles for enhancing clot formation by the manufacturer and silicone coated tubes to reduce red cell adherence to the tube wall), respectively. After allowing to clot for 20 minutes for the clot activator vacutainer and 40 minutes for the glass vial, both the collection tubes were centrifuged at 3,500 rpm for 10 minutes.
After proper centrifugation, both the collection tubes were immediately processed for serum Li estimation by VITROS 4600 analyzer in the laboratory within 1 hour of its collection. After processing, both the collection tubes were stored at 2 to 8°C without any aliquoting of serum sample separately and were again processed in the VITROS 4600 dry biochemistry analyzer, Ortho Clinical Diagnostics at 24 and 48 hours since the blood collection from the patient. Prioritizing the daily quality control procedures were followed as per the protocol. The clinical biochemistry laboratory also participated in the external quality control program of Bio-Rad where Li was one of the analytes along with other routine biochemical analytes reported by the laboratory.
For the study, an ethical clearance (IEC/2022/47) was obtained from the institute's ethics committee. Proper informed consent was taken from the patients, the study was conducted as per Declaration of Helsinki and confidentiality was maintained during this study. The statistical analysis was done by IBM SPSS software version 23.
Results
Of the 100 blood samples collected and analyzed in pairs, it was observed that blood sample collected in clot activator vacutainer had lower Li values in comparison to the glass vial collection method. The mean baseline “lithium” value by the glass vial collection method was 0.61 mmol/L and by the clot vacutainer collection method was 0.58 mmol/L. The percentage of coefficient of variance for Li was within satisfactory limits for both external and internal quality control program followed in the clinical biochemistry laboratory during study which were 3.7 and 3.5%, respectively.
The immediate observation of Li levels by both the collecting tubes was made within the 1st hour of blood collection. The mean of the difference of the serum Li levels by the two collection methods was found to be 0.04 with a standard deviation of 0.06 (►Table 1). The “t” test for the difference between the measurements of the Li levels was observed that the Li measurements by the two methods differed significantly with a confidence interval of 0.03 to 0.06 implying the level of agreement of both the methods is not similar (►Table 2).
| Sl. no. | Types of blood collection vials | Amount of blood collected in a vial | Any additives presenting the collecting blood vial |
|---|---|---|---|
| 1 | Clot activator vacutainer | 2.5 mL | Silica powder for enhancing clot formation and silicone coated to reduce red cell adherence to the tube wall |
| 2 | Plain glass vial | 2.5 mL | No additives |
| Mean difference | 95% CI of the difference | ||
|---|---|---|---|
| Lower | Upper | ||
| Measurement of lithium between glass vial and clot activator vacutainer within 1st hour of collection | 0.04 | 0.03 | 0.06 |
Abbreviation: CI, confidence interval.
To explore the extent and patterns of agreement, we created the Bland–Altman plot (►Fig. 1), with 95% statistical limits of agreement, represented by ± 1.96 standard deviations of the mean. The mean difference or the bias observed between the methods was 0.04 with a standard deviation of 0.06. The Bland–Altman plot clearly explains that there is good agreement between both the methods; the glass vial method can replace by clot activator method for serum Li estimation. Deming regression analysis of the values obtained from glass and clot activator vacutainer showed a good correlation, which states that there is no systemic difference between the two measurement methods so the glass vial method can replace the clot activator method of Li estimation (r = 0.9) (►Fig. 2).
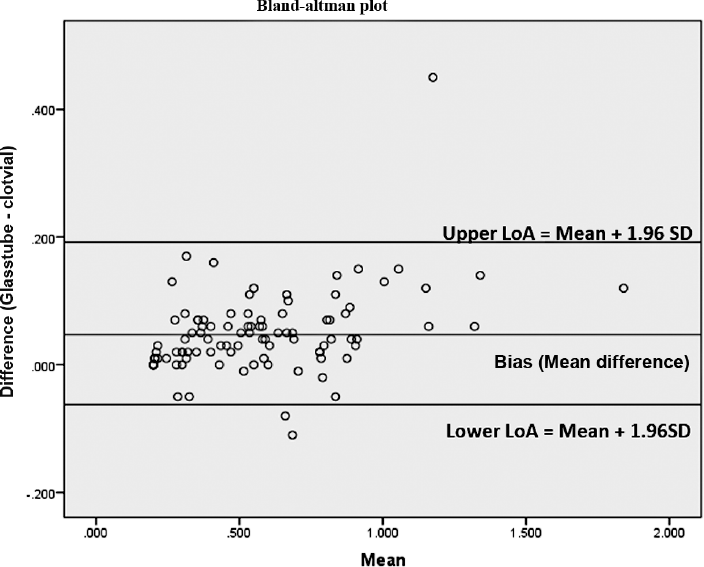
- The Bland–Altman Plot of 95% statistical limits of agreement (LoA), represented by ± 1.96 standard deviations (SD) of the mean. The mean difference or the bias observed between the methods was 0.04 with a SD of 0.0648 and the lower and upper limit of agreement spanning from −0.07 to 0.17 (mean ± 1.96SD).
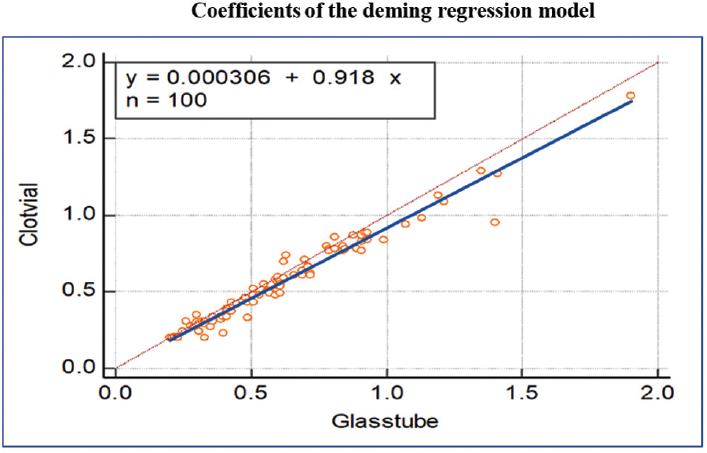
- The Deming regression showing the error measured during the analysis of values obtained from clot activator vacutainer and glass vial collection tube.
To check the stability of the Li samples in the collection vacutainers (glass vial and clot vacutainer), the samples were further processed and compared for Li values at 24 and 48 hours of the collection that was in addition to the baseline level at 1st hour of collection. The Li samples collected in clot activator vacutainer and glass vials were divided into three groups on the basis of time of Li estimations by the analyzer, that is, at 1st hour, 24 hours and 48 hours of the collection, which were tested statistically by one way analysis of variance (ANOVA) for differences across these groups. For both the glass vial and clot vacutainer collection methods (►Table 3), there was no statistical difference in the sample groups in different times after collection in ANOVA. Therefore, it can be said that all the groups were not different or same, that is, the Li levels do not change significantly at 1st hour, 24 hours, and 48 hours after collection.
| One-way ANOVA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Collection by clot activator vacutainer | Collection by glass vial | |||||||||
| Sum of squares | df | Mean square | F | Sig. | Sum of squares | df | Mean square | F | Sig. | |
| Between groups | 0.039 | 2 | 0.019 | 0.217 | 0.805 | 0.068 | 2 | 0.034 | 0.311 | 0.733 |
| Within groups | 26.474 | 297 | 0.089 | 32.347 | 297 | 0.109 | ||||
| Total | 26.512 | 299 | 32.415 | 299 | ||||||
Abbreviation: ANOVA, analysis of variance.
Discussion
With advanced technologies, the analytical phase of an individual test aims forward to be more accurate and precise. But the preanalytical phase always plays a pivotal role as it drives the analytical phase in the concise path of stringent quality control management of the clinical laboratory. So, in the present study, the clinical laboratory was a participant in BioRad external quality assurance program and had satisfactory performance in both external and internal quality controls. The therapeutic drug monitoring for serum Li is notably challenging for laboratory experts due to its multicomponent dependencies like the patient dosage prescription, last drug intake, time of sample collection, and types of collecting vacutainers used in the process of blood sample collection.
Serum Li, because of its narrow therapeutic range, that is, 0.6 to 1.2 mmol/L concentration, is monitored to ensure patient's compliance and to avoid Li overdose; thus, it is a prerequisite for an individual's dose adjustment in the treatment of bipolar mood disorder.[8]
In VITROS 4600 autoanalyzer is comprehensive tests facilities where the serum Li is estimated on dry slide platform assay using the reflectance photometry principle. The VITROS Li slide is a multilayered, analytical element coated on a polyester support. The Li in the sample is specifically bound by the crown-ether azo reagent (6-dodecyl-6-(2'-hydroxy-5′-(2”-4”-dinitrophenylazo) benzyl)-13,13-dimethyl-1,4,8,11-tetraoxacyclotetradecane) and forms a dye complex. As the Li ion binds to the crown-ether, a shift in the peak absorbance of the dye occurs. The increase in absorbance is proportional to the concentration of Li in the sample. The intensity of the dye is measured by reflectance spectrophotometry at 600 nm.9–11 The interaction of crown ether with a cation is based on the interaction of charge (the metal cation) and dipole (donor atom within the crown ether ring) that can be influenced by the orientation of the donor atom, cavity size of the crown ether, type of donor atom, the substitution of the electron donating, and withdrawing compounds and solvents.[12]
In comparison to other laboratories, serum Li is routinely estimated by ISE methods that work mainly on the principle of generation potential difference between the electrodes.[13]
Ikkurthi et al in their study have concluded that plain vacutainers with clot activators show higher bias and statistically significant higher levels of Li than glass vacutainers.[14] Thus, the study further validates the previous study by Sampson et al.[7] in which it was shown that silicone present as an additives in the clot activators vacutainer interacts with the membrane of Li ISE membrane. This interaction results in a false elevation of Li values when estimated on the ISE analyzers.
From the literature, Serdarević et al have compared the Li values obtained from the ISE method with VITROS dry chemistry method and atomic spectrophotometry method, where they have mentioned that there was a positive interference in the ISE method. The VITROS dry slide technology method provides reliable Li concentration results and is a valid alternative to atomic absorption spectrometry method.[15]
►Table 2 explains the decreased value of Li in the clot activator vacutainers that may be due to the presence of silica additives in the silicone-coated vacutainers that on exposure of the Li by dry chemistry analyzer results in decreased Li values. Crown ethers are well known for binding metal cations due to their cyclic cavities and electron donor features. Crown ethers are macrocyclic ligands containing ethyleneoxy units, so called polyethers. They have the ability to crown metal cations. Silicone particles(cations) present in the clot activator vacutainers may have interfered with the crown ethers resulting in decreased value of serum Li.[12]
Vacutainers supplied by different manufacturers vary in the materials and additives that can potentially affect the test performance. Silicone surfactant-coated vacutainers have been shown to interfere with ion-specific electrode measurement of not only Li but also of ionized magnesium through interaction with ion-specific electrode membranes leading to increase in the measured voltage.[16] Sampson et al showed that silica and silicone surfactant are associated with elevated Li concentrations when using the Lytening 2Z (Lytening West Peabody, Massachusetts, United States) ion-specific electrode analyzer.[6]
This confirms that the value obtained from the glass vacutainers is more reliable for patient therapeutic drug monitoring as no such additives are present in the glass vacutainers. In our study, we have also found that there was no statistical difference in the stability of serum Li values processed from the glass vial and clot activator vacutainer, 24 hours and 48 hours of collection. This might be because of proper storage and processing of the samples at the optimum temperature and the better estimation method as compared with the ISE. But another study has advocated Li estimation to be performed within 6 hours as its level falls after that and they have also attributed it to a change in pH affecting the cationic assays,[17] which might be one of the causes for the reduced Li value estimation on different time interval.
Conclusion
In this study, Li levels measured in the clot activated vacutainers were lower as compared with Li levels measured in the glass vials. There is no apparent change in Li levels at 1st hour, 24 hours, and 48 hours after the collection of blood samples collected in both the glass vacutainer and clot-activated method when measured by the VITROS 4600 dry chemistry analyzer. Collections of blood specimen for Li estimation should be preferred in glass vial in comparison to the clot activator vacutainer. There were apparently lower Li levels in the red capped clot activator tube due to the interference of silicone coated particles that is inherently present in the vacutainer for enhancing clot formation process for better extraction of serum, within 10 to 15 minutes of collection of blood.
Within limited resources, this study provides satisfactory evidence on the reflectance photometry technique to justify the difference in the Li values from the analysis of serum collected by glass vial in comparison to the red capped clot activator vacutainer. Li estimation in routine is done in a laboratory as a part of TDM, but because of its narrow therapeutic reference range, it is a challenge for the clinical laboratory to ascertain accuracy. As blood samples collected in different collection tubes have documented interference because of silicone coated tubes, the use of glass vial should be preferred. In the future, comparative studies and cost-effective analysis can be performed using glass vial, clot activator, and heparinized vacutainers for choosing the best method as per the demand of the laboratory.
Authors' Contributions
All the authors have equally contributed to the accomplishing of the study.
Conflict of Interest
None declared.
References
- The treatment of manic psychoses by the administration of lithium salts. J Neurol Neurosurg Psychiatry. 1954;17(04):250-260.
- [CrossRef] [PubMed] [Google Scholar]
- Overview of therapeutic drug monitoring. Korean J Intern Med (Korean Assoc Intern Med). 2009;24(01):1-10.
- [CrossRef] [PubMed] [Google Scholar]
- NIMH/NIH Consensus Development Conference statement. Mood disorders: pharmacologic prevention of recurrences. Am J Psychiatry. 1985;142(04):469-476.
- [CrossRef] [PubMed] [Google Scholar]
- Lithium. In: Schumacher GE. ed. Therapeutic Drug Monitoring. Norwalk, CT: Appleton & Lange; 1995:493-526.
- [Google Scholar]
- Clinical laboratory requirements for lithium assays and future of lithium assays. Trace Elem Electrolytes. 2007;24:6-12.
- [CrossRef] [Google Scholar]
- Positive interference in lithium determinations from clot activator in collection container. Clin Chem. 1997;43(04):675-679.
- [CrossRef] [PubMed] [Google Scholar]
- Interference in serum lithium estimation by silica clot activator and silicone surfactant in ISE principle: a cross-sectional study. Bangladesh Journal of Medical Biochemistry. 2017;8(02):60-65.
- [CrossRef] [Google Scholar]
- Vitros Chemistry Products calibrator Kit 1, Instruction for use, J11322_EN, Version 13.0.
- [Google Scholar]
- Thermodynamic study of crown ether-lithium/magnesium complexes based on benz-1,4-dioxane and its homologues. Phys Chem Chem Phys. 2022;24(19):11687-11695.
- [CrossRef] [PubMed] [Google Scholar]
- Routines and challenges in clinical application of electrochemical ion-sensors. Electroanalysis. 2014;26(06):1171-1181.
- [CrossRef] [Google Scholar]
- A comparative evaluation of lithium estimation for samples collected in different tubes and its stability on storage. J Lab Physicians. 2018;10(01):56-59.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of Vitros dry slide technology for determination of lithium ions with other methods. Bosn J Basic Med Sci. 2006;6(02):32-36.
- [CrossRef] [Google Scholar]
- Interferences from blood collection tube components on clinical chemistry assays. Biochem Med (Zagreb). 2014;24(01):31-44.
- [CrossRef] [PubMed] [Google Scholar]






