Translate this page into:
A comparative in vitro sensitivity study of “cefepime-tazobactam” and other antibiotics against Gram-negative isolates from intensive care unit
*Corresponding author: Chinmoy Sahu, Department of Microbiology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, Uttar Pradesh, India. csahu78@rediffmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kar M, Siddiqui T, Sengar S, Sahu C. A comparative in vitro sensitivity study of “cefepime-tazobactam” and other antibiotics against Gram-negative isolates from intensive care unit. J Lab Physicians. 2024;16:194-199. doi: 10.25259/JLP-16-194-(1769)
Abstract
Objectives:
The major health problem today includes the emergence of multidrug-resistant (MDR) bacteria, especially extended-spectrum β lactamase, carbapenemases, and Amp C-producing Gram-negative bacilli (GNB). Our study is aimed to recognize the in vitro susceptibility pattern of cefepime/tazobactam compared to other antibiotics used against GNB in an intensive care unit (ICU) setting.
Materials and Methods:
We conducted a prospective observational research comprising all GNB isolated from clinical samples of patients admitted to the ICU throughout the study period from January 2021 to December 2021. All of the isolates were analyzed using “ Matrix Assisted Laser Desorption/ionization - time of flight - Mass spectrometry assay (MALDI-TOF-MS)” for identification and the Kirby-Bauer disc diffusion technique to test for susceptibility. Cefepime-tazobactam was tested by E-test (Hi-Media, Mumbai) method. The minimum inhibitory concentrations (MIC) of cefepime (as in Clinical Laboratory Standards Institute, 2021) has been utilized to elucidate the sensitivity of cefepime-tazobactam, as no criteria for cefepime-tazobactam is available.
Statistical Analysis:
All statistical analysis was performed using the software program IBM Statistical Package for Social Sciences (SPSS) Statistics version 20.0, with P < 0.05 considered statistically significant.
Results:
We included a total of 480 GNB isolated from blood, pus, body fluids, endotracheal aspirates (ETA), and sputum samples. The most common microorganism tested for susceptibility to cefepime-tazobactam was Klebsiella pneumoniae (182/480, 37.92%) followed by Acinetobacter baumannii (135/480, 28.12%), and Pseudomonas aeruginosa (94/480, 19.58%). K. pneumoniae and Enterobacter aerogenes were most resistant to all the antibiotics tested against them. K. pneumoniae was most resistant to meropenem (41/182, 22.53%), followed by imipenem (42/182, 23.08%) and cefoperazone-sulbactam (49/182, 26.92%) and was predominantly found susceptible to cefepime-tazobactam (122/182, 67.04%).
Conclusions:
Cefoperazone-tazobactam is a new “β-lactam/β-lactamase combination” found effective in the in vitro analysis of drug-resistant isolates of GNB.
Keywords
Carbapenemases
Cefepime
Cefoperazone-sulbactam
Cefepime-tazobactam
MALDI-TOF-MS
INTRODUCTION
The indiscriminate use of third and fourth -generation cephalosporin and carbapenems in the past few decades has led to widespread antibiotic resistance among Gram (−ve) bacteria; Thereby restricting the use of several classes of antibiotics and using the antibiotic of last resort, Colistin.[1,2] Recently, some microorganisms have developed resistance to colistin leading to pan-drug resistant infections with an imperative demand for newer antibiotics.[3-5] Beta-lactam resistance has been encountered as the most common form of resistance encountered among the microorganisms. The use of Beta-lactam/Beta-lactamase inhibitor is a better method to counter the resistance to Beta-lactams.[6,7]
Among Indian hospitals, there is high resistance to extended-spectrum β lactamases (ESBLs), and a similar trend is being observed in carbapenems due to irrational use in most critical care units.[8,9] Thus, an ever-increasing menace of Gram-negative antibiotic resistance is being encountered in Indian healthcare, which can be attributed to the indiscriminate usage of antibiotics, over-the-counter availability of all antibiotic agents, and the absence of national antibiotic policy to counter the skyrocketing antimicrobial resistance.[10]
The discovery of new antibiotics is the need of the hour to counter the ever-increasing antibiotic resistance. A fourth-generation cephalosporin with a β-lactamase inhibitor combination provides coverage to OXA and AmpC enzymes over third -generation cephalosporins.[7] Among newer BL/BLI combinations, cefepime/tazobactam is a new drug approved for use in Indian hospitals by the Drug Controller General of India.[11] No clinical data is available in the literature for this drug combination, and very limited studies have been published on this drug combination to date. The aim of the research was to elucidate the in vitro sensitivity of “Cefepime-tazobactam” and some other antibiotics against Gram (−ve) isolates from the intensive care unit (ICU).
MATERIALS AND METHODS
Duration and place of study
This prospective work has been done in the “Bacteriology Section” of the microbiology department at our university hospital from January 2021 to December 2021.
Sample collection
During the study period, all non-duplicate Gram-negative bacilli (GNB) isolates obtained from all clinical samples including blood, pus, body fluids, endotracheal aspirates (ETA), and sputum were taken from the ICU patients and send to “Bacteriology Section” of Laboratory of Microbiology department.
Inclusion criteria
As multiple samples were sent from a single patient admitted to the ICU, we included only the first sample growing GNB from a single patient. Thus, non-repeat samples were included in this study [Figure 1].
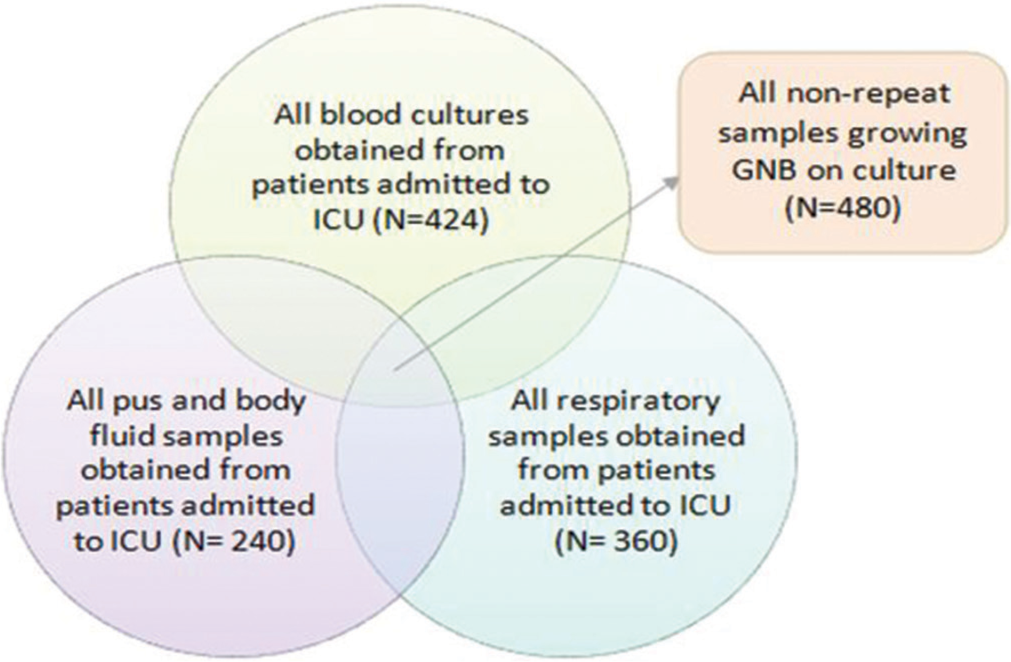
- Inclusion of the pathogenic GNB isolated from various samples obtained from patients admitted to the ICU (n = 1024). GNB: Gram negative bacteria, ICU: Intensive care unit, N: Number of samples.
Exclusion criteria
All samples coming to the laboratory after 2 h from sample collection, growing contaminants, and commensals were excluded from the study.
Characterization of samples
The production for the Screening of ESBL and Metallo-β-lactamase (MBL) was performed on all the GNB isolates respecting Clinical Laboratory Standards Institute (CLSI) guidelines.[12] ESBL production possibly was demonstrated by isolates zone size “≤22 for ceftazidime (30 μg), ≤27 with cefotaxime (30 μg), and ≤25 with ceftriaxone (30 μg”). Disc potentiation test has been performed using “cefotaxime (30 μg) and ceftazidime (30 μg) antibiotic disk with or without clavulanic acid (10 μg)” and by “double disk susceptibility test” has been used to confirm the production of ESBL.[12] Yong et al.[13] used imipenem (10 μg) only and imipenem (10 μg) along with ethylenediaminetetraacetic acid (750 μg) disk for phenotypic MBL detection in clinical isolates.
Antimicrobial susceptibility testing
As recommended by the CLSI guidelines (2019),[12] Kirby-Bauer disc diffusion test was employed to carry out an antibiotic susceptibility test. The disk of “piperacillintazobactam” (100/10 μg), cefepime (30 μg), “cefoperazonesulbactam” (75/30 μg), 10 μg of meropenem, ertapenem and imipenem, and E-strips of cefepime-tazobactam were taken from HiMedia (Mumbai, India). The zone diameters were interpreted as susceptible, intermediate, or resistant respecting CLSI guidelines. As any criteria for interpretation of zone diameter of cefepime-tazobactam and cefoperazonesulbactam were not available, to evaluate the susceptibility of these two medication combinations, cefepime and cefoperazone zone size as per CLSI 2016 were utilized.[14]
For cefepime-tazobactam (30/10 μg) to be susceptible, a ≥25 for Enterobacteriaceae and ≥18 zone size for nonfermenters was considered. Quality control of every disc was done according to “CLSI 2016”[14] approved disk diffusion QC ranged against “Pseudomonas aeruginosa ATCC 27853, Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 25923, and Klebsiella pneumoniae ATCC 700603.”
RESULTS
We included 480 total GNB isolated from blood, pus, body fluids, ETA, and sputum samples in our study cohort. The maximum number of GNB isolates tested for susceptibility to cefepime-tazobactam was obtained from endotracheal aspirate samples (120/480, 25%), followed by those isolated from bloodstream infections (115/480, 23.95%) and sputum (112/480, 23.33%), as shown in Figure 2.
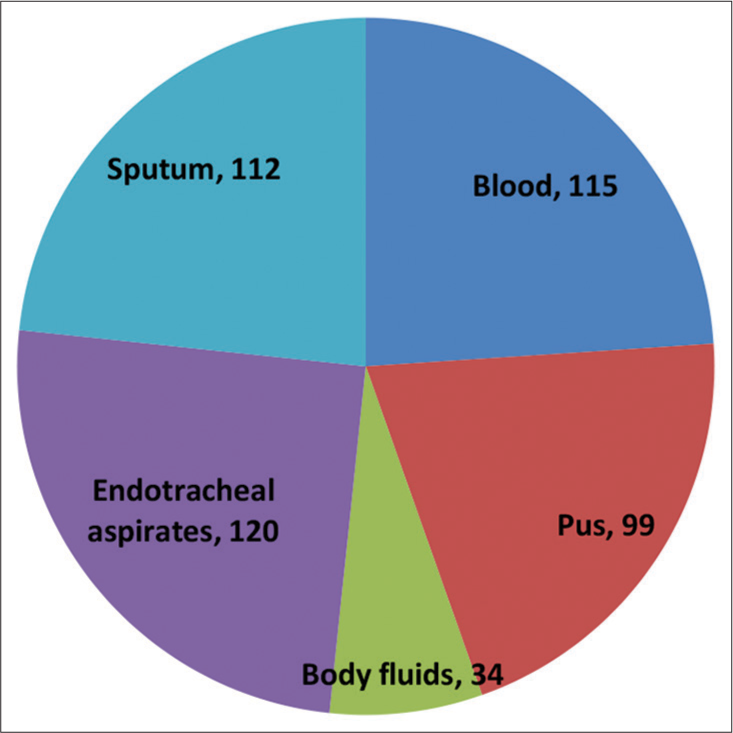
- The distribution of all the samples included in the study cohort (n = 480). n: Number of samples.
The most common microorganism tested for susceptibility to cefepime-tazobactam was K. pneumoniae (182/480, 37.92%) followed by Acinetobacter baumannii (135/480, 28.12%) and P. aeruginosa (94/480, 19.58%). Figure 3 describes the distribution of the microorganisms from the various samples included in the study. The most common microorganism was K. pneumoniae, which was mostly isolated from endotracheal aspirate (75/182, 41.21%) followed by bloodstream infections (69/182, 37.91%). A. baumannii was isolated most commonly from ETA (68/135, 50.37%) followed by sputum samples (42/135, 31.11%), while P. aeruginosa was isolated mostly from endotracheal aspirate samples (43/94, 45.74%).
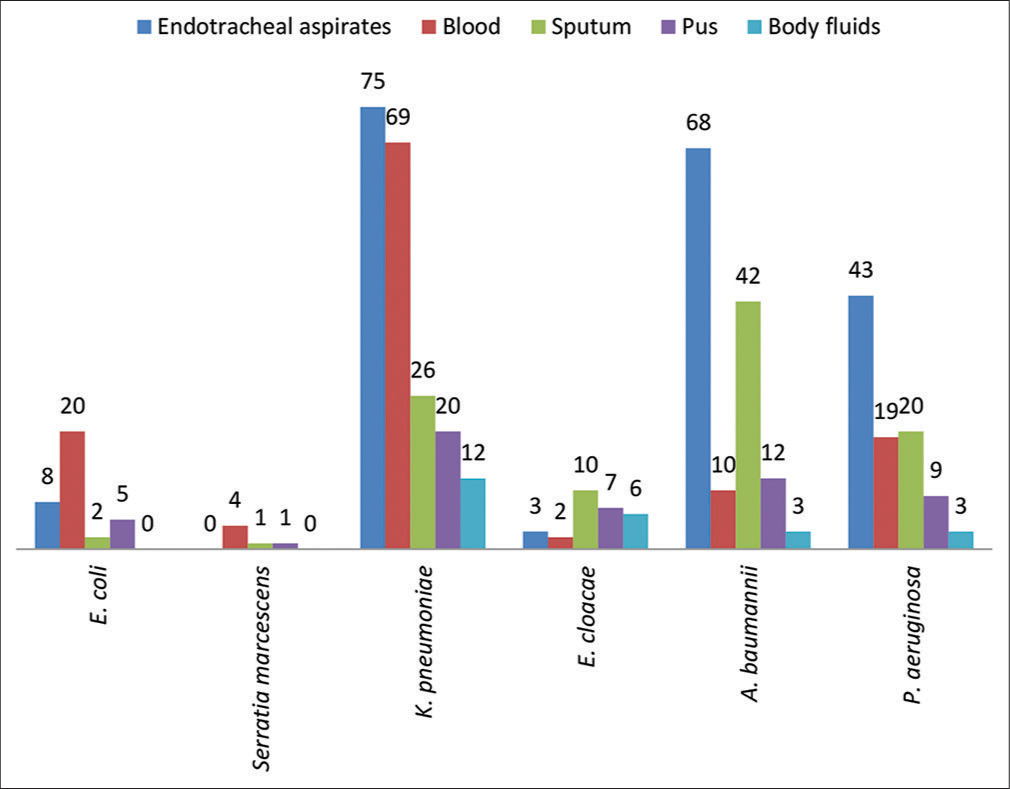
- The distribution of pathogenic microorganisms isolated from various samples included in this study (n = 480). n: Number of samples. E.coli: Escherichia coli, K.pneumoniae, Klebsiella pneumonia, E.cloacae: Enterobacter cloacae, A.baumannii: Acinetobacter baumannii, P. aeruginosa, Pseudomonas aeruginosa.
The microorganisms were further divided into two groups, namely, non-inducible Enterobacteriaceae group (which includes E. coli and K. pneumoniae) and the inducible Enterobacteriaceae group (which includes Enterobacter aerogenes and Serratia marcescens) and those belonging to non-inducible Enterobacteriaceae group (n = 217, 45.21%) were predominantly tested for susceptibility to cefepimetazobactam in comparison to inducible Enterobacteriaceae group (n = 36, 7.5%). We also analyzed the results of antibiotic susceptibility testing with cefepime-tazobactam and other first-line drugs against the isolates included in the study [Table 1]. The drug in question was also tested for susceptibility against non-fermenters (n = 229, 47.71%).
| Antibiotics | Cefepime (%) | Cefepime-tazobactam (%) | Cefoperazone-sulbactam (%) | Imipenem (%) | Meropenem (%) |
|---|---|---|---|---|---|
| Non-inducible Enterobacteriaceae (n=217) | |||||
| Escherichia coli (n=35) | 11/35 (31.43) | 26/35 (74.28) | 10/35 (28.57) | 8/35 (22.86) | 7/35 (20.0) |
| Klebsiella pneumoniae (n=182) | 50/182 (27.47) | 122/182 (67.04) | 49/182 (26.92) | 42/182 (23.08) | 41/182 (22.53) |
| Inducible Enterobacteriaceae (n=34) | |||||
| Enterobacter cloacae (n=18) | 5/18 (27.78) | 11/18 (61.11) | 2/18 (11.11) | 3/18 (16.67) | 3/18 (16.67) |
| Serratia marcescens (n=16) | 12/16 (75.0) | 16/16 (100.0) | 12/16 (75.0) | 15/16 (93.75) | 14/16 (87.5) |
| Non-fermenters (n=229) | |||||
| Acinetobacter baumannii (n=135) | 34/135 (25.18) | 89/135 (65.92) | 32/135 (22.22) | 31/135 (22.96) | 30/135 (22.22) |
| Pseudomonas aeruginosa (n=94) | 25/94 (26.59) | 58/94 (61.70) | 22/94 (23.40) | 21/94 (22.34) | 21/94 (22.34) |
| All isolates (n=480) | 137/480 (28.54) | 322/480 (67.08) | 127/480 (26.46) | 120/480 (25.00) | 116/480 (24.17) |
n: Number of samples.
Table 1 also represents the antibiotic susceptibility of all Gram (−-ve) isolates to cefepime, cefoperazone-sulbactam, cefepime-tazobactam, meropenem, and imipenem. Serratia marcescens was the most susceptible isolate to all the antibiotics and was 75.0%–100.0% (12/16, 75.0% to 16/16, 100.0%) susceptible to all the five antibiotics. K. pneumoniae and E. aerogenes were most resistant to all the antibiotics tested against them. K. pneumoniae was most resistant to meropenem (41/182, 22.53%), followed by imipenem (42/182, 23.08%) and cefoperazone-sulbactam (49/182, 26.92%), and was predominantly found susceptible to cefepime-tazobactam (122/182, 67.04%). E. aerogenes was least susceptible by cefoperazone-sulbactam (2/18, 11.11%), followed by both carbapenems (3/18, 16.67%) each and cefepime (5/18, 27.47%) and primarily found susceptible to cefepimetazobactam (11/18, 61.11%). Overall, susceptibility to all the antibiotics in descending order was as follows: cefepimetazobactam (322/480, 67.08%), cefepime (137/480, 28.54%), cefoperazone-sulbactam (127/480, 26.46%), imipenem (120/480, 25.0%), and meropenem (116/480, 24.17%).
Table 2 describes the comparative activity of cefepimetazobactam and cefepime against the most common three microorganisms isolated from the samples involved in the present study. K. pneumoniae has been identified as the most susceptible (50/182, 27.47%) and most resistant (60/182, 32.96%) microbe to both the cefepime and cefepime-tazobactam. Seventy-two (72/182, 39.56%) isolates of K. pneumoniae were found susceptible to cefepime-tazobactam but were resistant to cefepime, while none of the isolates were found susceptible to cefepime and resistant to cefepime-tazobactam. A. baumannii (55/135, 40.74%) was found to be most susceptible to cefepimetazobactam but was resistant to cefepime.
| Microorganisms | Susceptible to both cefepime and cefepime-tazobactam (%) | Resistant to both cefepime and cefepime-tazobactam (%) | Sensitive to cefepime-tazobactam and resistant to cefepime (%) | Resistant to cefepime-tazobactam and sensitive to cefepime (%) |
|---|---|---|---|---|
| Klebsiella pneumoniae(n=182) | 50 (27.47) | 60 (32.96) | 72 (39.57) | 0 (0.0) |
| Acinetobacter baumannii (n=135) | 34 (25.18) | 46 (34.07) | 55 (40.74) | 0 (0.0) |
| Pseudomonas aeruginosa (n=94) | 25 (26.59) | 36 (38.29) | 33 (35.11) | 0 (0.0) |
n: Number of samples.
DISCUSSION
There is serious global concern regarding the rise of antibiotic resistance in recent times,with few to no newer antibiotics in the pipeline. With the advent of increasing Carbapenem resistance, there is an increasing interest in new combinations like “cefepime-tazobactam.” Cefepime is a fourth generation, synthetic cephalosporin with broad-spectrum action.[15] The antibiotic exhibits better action against the Gram (−ve) bacteria in comparison to the Gram-positive bacteria. Tazobactam is a beta-lactamase that inhibits the activity of the beta-lactamase enzymes including “group 1-cephalosporinases, group 2br-TEM beta-lactamases, and group 3-metallo-beta-lactamases.” “Cefepime-tazobactam” is expected to overcome ESBL, AmpC, and OXA enzyme production.[16,17]
There is a lack of studies comparing the efficacy of drugs most commonly used in the ICU, which include a variety of cephalosporins, carbapenems, and BL/BLI combinations. This study reports a better susceptibility of the Gram (−ve) bacteria to cefepime-tazobactam in comparison to carbapenems, and other cephalosporins that are profusely used in the ICU setup, which agrees with research done by Sood et al.[17] and Mushtaq et al.[18] The common microorganisms isolated from various samples such as K. pneumoniae, A. baumannii, and P. aeruginosa showed better susceptibility to cefepime-tazobactam in comparison to cefepime alone. Therefore, similar findings were stated by Susan et al.,[6] and other authors’[17-19].
In the wake of increasing carbapenem resistance of ~ 25% susceptibility of the commonly isolated microorganisms reported in our study, the susceptibility of the microorganisms was better to cefepime-tazobactam (67.08%). This is in comparison to the research by Agarwal et al.[15] where carbapenems showed comparable performance to cefepime-tazobactam. A similar study performed in patients with hematological malignancies by Benanti et al.[20] also showed comparable results with both the antibiotics. However, the patients were shifted back on carbapenems early in the treatment. Other Indian studies by Ghafur et al.[21] and Sharma et al.[22] reported either equitable or better susceptibility of the microorganisms to both carbapenems and cefepime-tazobactam which are contradictory to our finding of better susceptibility of the organisms to cefepime-tazobactam. A similar rising trend of carbapenem resistance is noticed in research by Vu et al.[23] that further compels us to identify new antibiotics to combat the drug resistance among the isolates. Vu et al.[23] supported the use of cefepime-tazobactam in place of carbapenems to prevent the rapid emergence of drug resistance among commonly isolated microorganisms from the ICU settings.
The main limitations of the present analysis are: primary, it is a single-center study and does not represent the rate of drug resistance in a particular geographical region: secondary, due to the unavailability of any zone diameters for the antibiotics susceptibility of cefepime-tazobactam and no specific CLSI guidelines for the same, we had to improvise using the disc diameters of cefepime recommended in CLSI.
CONCLUSIONS
Our study defines a good in vitro activity of “cefepimetazobactam” against non-inducible, inducible, and nonfermenters in comparison to other BL/BLI combinations and carbapenems used routinely in ICUs. It is a potential antimicrobial agent for treating an array of Gram-negative bacteria-associated infections, and it improves the outcome of patients suffering from infections caused by GNB in comparison to other antimicrobial agents.
Ethical approval
The study has been approved by the institutional ethics committee of Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow with the ethical approval number: 2021-48-EMP-EXP, dated 29/11/2021.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Outcome of infections due to pan drug-resistant (PDR) Gram-negative bacteria. BMC Infect Dis. 2005;5:24-8.
- [CrossRef] [PubMed] [Google Scholar]
- Empiric use of Cefepime in treatment of lower respiratory tract infections in children. Paed Infect Dis J. 2001;20:543-9.
- [CrossRef] [PubMed] [Google Scholar]
- Outbreak of colistin-resistant, carbapenem-resistant Klebsiella pneumonia in metropolitan detroit, Michigan. Antimicrob Agent Chemother. 2011;55:2593-9.
- [CrossRef] [PubMed] [Google Scholar]
- Emergence of tigecycline and colistin resistant Acinetobacter baumanii in patients with complicated urinary tract infections in north India. Indian J Med Res. 2011;133:681-4.
- [Google Scholar]
- β-Lactam/β-Lactam inhibitor combinations for the treatment of bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli A post-hoc analysis of prospective cohorts. Clin Infect Dis. 2012;54:167-74.
- [CrossRef] [PubMed] [Google Scholar]
- A comparative in vitro study of Cephalosporin/Beta-lactamase inhibitor combinations against Gram-negative bacilli. Indian J Physiol Pharmacol. 2013;57:425-31.
- [Google Scholar]
- Orthodox and unorthodox clavulanate combinations against extended-spectrum ß-lactamase producers. Clin Microbiol Infect. 2008;14:189-93.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of ESBL producers amongst gram-negative bacilli isolated from intra-abdominal infections across India (based on SMART study, 2007 data) J Assoc Physicians India. 2011;59:287-92.
- [Google Scholar]
- Can India be the wing commander in the global fight against antimicrobial resistance? J Assoc Physicians India. 2012;60:42-3.
- [Google Scholar]
- Clinical profile of patients treated with cefepime/tazobactam: A new ß-lactam-ß-lactamase inhibitor combination. J Microbiol Infect Dis. 2012;2:79-86.
- [CrossRef] [Google Scholar]
- Performance standards for antimicrobial susceptibility testing: Twenty-third informational supplement In: CLSI document M100-S29. Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2019.
- [Google Scholar]
- Imipenem-EDTA disk method for differentiation of Metallo-β-lactamase-producing clinical isolates of Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol. 2002;40:3798-80.
- [CrossRef] [PubMed] [Google Scholar]
- Performance standards for antimicrobial susceptibility testing; 26th informational supplement In: CLSI document M100-S26. Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2016.
- [Google Scholar]
- Cefepime/tazobactam as a new treatment option for multidrug-resistant gram-negative bacilli. Int J Res Med Sci. 2019;7:2278.
- [CrossRef] [Google Scholar]
- Cephalosporins In: Mandell GL, Bennett JE, Dolin R, eds. Mandell Douglas and Bennetts’ principles and practice of infectious diseases (7th ed). United Kingdom: Churchill Livingstone; 2010. p. :323-36.
- [CrossRef] [Google Scholar]
- Comparative evaluation of the in-vitro activity of six β-lactam/β-lactamase inhibitor combinations against gram-negative bacilli. J Clin Diagn Res. 2013;7:224-8.
- [CrossRef] [PubMed] [Google Scholar]
- Cefepime/tazobactam compared with other tazobactam combinations against problem Gram-negative bacteria. Int J Antimicrob Agents. 2021;57:106318.
- [CrossRef] [PubMed] [Google Scholar]
- Antimicrobial activity of high-dose cefepime-tazobactam (WCK 4282) against a large collection of Gram-negative organisms collected worldwide in 2018 and 2019. Int J Infect Dis. 2022;116:306-12.
- [CrossRef] [PubMed] [Google Scholar]
- Carbapenem versus cefepime or piperacillin-tazobactam for empiric treatment of bacteremia due to extended-spectrum-β-lactamase-producing Escherichia coli in patients with hematologic malignancy. Antimicrob Agents Chemother. 2019;63:e01813-8.
- [CrossRef] [PubMed] [Google Scholar]
- Sensitivity pattern of Gram-negative bacteria to the new β-lactam/β-lactamase inhibitor combination: Cefepime/tazobactam. J Microbiol Infect Dis. 2012;2:5-8.
- [CrossRef] [Google Scholar]
- Cefepime tazobactam: A new β lactam/β lactamase inhibitor combination against ESBL producing gram-negative bacilli. Int J Pharm Biomed Sci. 2012;2:35-8.
- [Google Scholar]
- Re-evaluation of cefepime or piperacillintazobactam to decrease use of carbapenems in extended-spectrum beta-lactamase-producing Enterobacterales bloodstream infections (REDUCE-BSI) Antimicrob Steward Healthc Epidemiol. 2022;2:e39.
- [CrossRef] [PubMed] [Google Scholar]






