Translate this page into:
A Simple, Rapid, and Cost-Effective PCR Procedure for Detection of NUDT15 Gene Variants in Vietnamese Patients with Acute Lymphoblastic Leukemia
Address for correspondence: Hoang Anh Vu, MD, PhD, Center for Molecular Biomedicine, University of Medicine and Pharmacy at Ho Chi Minh City, 217 Hong Bang Street, District 5, Ho Chi Minh City, Vietnam (e-mail: hoanganhvu@ump.edu.vn).
-
Received: ,
Accepted: ,
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective
The NUDT15 variants impact thiopurine dose selection in acute lymphoblastic leukemia patients. The ability to rapidly detect variants is important in clinical practice. This study aims to develop a simple polymerase chain reaction (PCR) procedure for detecting NUDT15 variants in Vietnamese patients.
Materials and Methods
Sanger sequencing was used to determine NUDT15 variants from 200 patients. We designed primers and optimized the PCR procedure for detection of wild-type and variant alleles and compared with Sanger sequencing results.
Results
The inserted variant c.55_56insGAGTCG was detected by differences in size through conventional PCR. The tetra-primer amplification refractory mutation system PCR was successful in detecting two variations, c.52G > A and c.415C > T. The sensitivity and specificity of PCR procedure achieved 100% when compared to 200 Sanger sequencing results.
Conclusion
Our PCR procedure is suitable for replacing Sanger sequencing to detect the NUDT15 variants in clinical setting.
Keywords
NUDT15
tetra-primer ARMS PCR
gene variant
Vietnamese
Introduction
Located in Southeast Asia, Vietnam has a population of about 100 million. There were more than 350,000 cancer patients in Vietnam in 2020, with over 182,000 new cases and 122,000 deaths of cancer yearly. Acute lymphoblastic leukemia (ALL), a malignant transformation and proliferation of lymphoid progenitor cells in the bone marrow, blood, and extramedullary sites,[1] accounts for approximately 30% of all pediatric cancer and is the most common form of leukemia in children. ALL treatment's primary goals are to achieve complete remission and to maintain long-term remission over a 5-year period.[2] Thiopurines (e.g., 6-mercaptopurine [6-MP]) play a critical function in ALL treatment and they are related to drug-induced myelosuppression.[3] During cell replication, 6-MP is converted into thiopurine nucleotides, then attaches continuously to deoxyribonucleic acid (DNA) and leads to cell cytotoxicity.[4] Thiopurine methyltransferase (TPMT) is the first described enzyme linked to 6-MP intolerance, and its decreased enzyme activity is related to increased drug toxicities.[5]TPMT gene variants are common in the Western world.[6] In 2015, a study reported a variant in the NUDT15 gene associated with 6-MP intolerance in ALL.[7] In contrast with TPMT gene, polymorphisms in the NUDT15 gene are more common in Asian populations.[6] Clinical trials have indicated that variants including p.Val18Ile (c.52G > A), p.Val18_Val19insGlyVal (c.55_56insGAGTCG), and p.Arg139Cys (c.415C > T) impact thiopurine metabolism and tolerance.[8]
Previous studies used Sanger sequencing, which was considered to be the gold standard for detecting NUDT15 variants. We have recently studied 200 ALL patients using Sanger technique to determine the percentage of NUDT15 variants and their effects on the 6-MP treatment for pediatric ALL (data submitted). However, Sanger sequencing has some disadvantages such as expensive, complicated, and time-consuming. Therefore, we want to design this study in such a way that the variant identification approach can be generally used in clinical practice in Vietnam. This polymerase chain reaction (PCR)-only technique has the advantages of being inexpensive, requiring less time to detect variants, and being applicable in basic laboratories.
Materials and Methods
Samples
In a previous study, we used Sanger sequencing method to detect the NUDT15 gene variants as determinant factors of 6-MP treatment on 200 ALL patients (data submitted). In the present study, DNAs from previous study were used to develop a single PCR procedure for detection of known variants. For specificity and sensitivity, the PCR results were compared to Sanger sequencing data.
The protocol for this study was approved by the Ethics Committee of the University of Medicine and Pharmacy at Ho Chi Minh City, Vietnam (approval number 244/HDDD-DHYD).
Primer Design
Primers for PCR were designed to detect 3 variants, including 2 variants on the exon 1, which are c.55_56insGAGTCG and c.52G > A, and variant c.415C > T on the exon 3 based on the referenced genomic sequence of NUDT15 (NG_047021.1 in GenBank).
To detect the c.55_56insGAGTCG, a pair of primer for conventional PCR was designed to amplify the region containing this variant, with a normal allele product of 75 base pair (bp) (NUD-insF: 3′-CTATGACGGCCAGCGCAC-5′; NUD-insR: 3′-GCTTGCAGCTGGTCACCA-5′). The presence of an extra band with 6 bp higher than normal band (a band with product of 81 bp) identifies the inserted variant allele.
For the c.52G > A variant, a set of 4 primers was designed based on tetra-primer amplification refractory mutation system (ARMS) PCR criteria to detect the normal and variant alleles in homozygous or heterozygous forms (►Fig. 2A). A pair of 2 outer primers (forward, NUD-1F1 and reverse, NUD-1R2) was designed to amplify the region containing this variant with product of 176 bp. To detect the variant allele, the forward outer primer was used with a reverse inner primer (NUD-52mR) for amplifying a product of 120 bp. Because different mismatches have various destabilizing effects, when designing the primer, both the terminal and penultimate mismatches have to be considered together.[9] The NUD-52mR was designed with “T” nucleotide at 3′-end and a deliberate mismatch at third position from the 3′-end (“A” instead of “G” nucleotide) so that it differs from both the normal and variant alleles.[10] To detect the normal allele, a forward inner primer was designed with “C” nucleotide at 3′-end, and used in combination with the reverse outer primer (NUD-52wtF and NUD-1R2) for detecting normal allele with product of 76 bp. From in silico prediction, for the NUDT15 c.52G > A genotyping, three product bands of 176 bp (common amplicon), 76 bp (wild-type/C allele-specific amplicon), and 120 bp (variant/T allele-specific amplicon) were expected for heterozygous variant; two bands of 176 and 120 bp were expected for homozygous; and two bands of 176 and 76 bp were expected for wild-type.
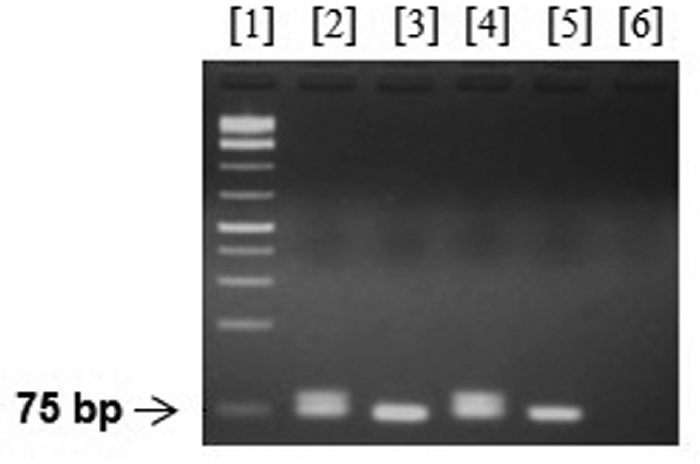
- Genotyping of NUDT15 c.55_56insGAGTCG variant by conventional polymerase chain reaction (PCR). Wild-type and variant status were distinguished by differences in size on electrophoresis. Lane 1, GeneRuler 1 kp Plus DNA Ladder (Thermo Fisher); lanes 2 and 4, samples from patients having NUDT15 variant with extra 6 base pair (bp); lanes 3 and 5, samples from patients without NUDT15 variant; lane 6, distilled water.
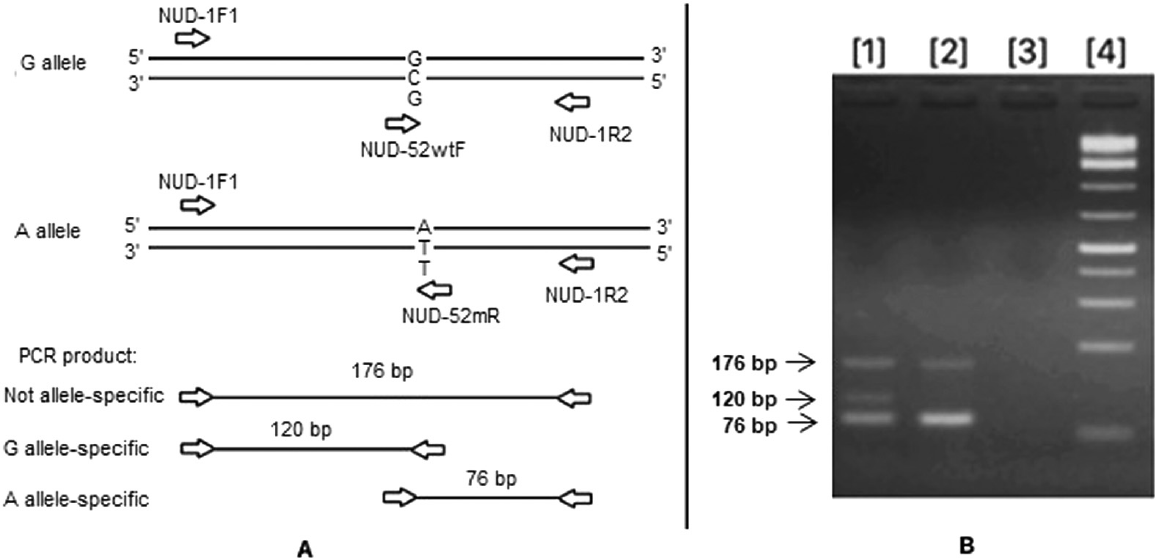
- Genotyping of NUDT15 c.52G > A variant. (A) Schematic illustration of primer design for the tetra-primer amplification refractory mutation system (ARMS) polymerase chain reaction (PCR). (B) Tetra-primer ARMS PCR for detecting c.52G > A variant. Lane 1, sample of patient carrying heterozygous variant (G/A); lane 2, sample of patient without variant (G/G); lane 3, distilled water; lane 4, GeneRuler 1 kp Plus DNA Ladder (Thermo Fisher).
Similarly, for the c.415C > T variant, a set of 4 primers was designed based on tetra-primer ARMS PCR criteria (►Fig. 3A). A pair of 2 outer primers (NUD-3F1 and NUD-3R1) was designed to amplify the region containing this variant with product of 233 bp. A pair of forward inner primer and reverse outer primer (NUD-MUF and NUD-3R1) was used for detecting variant allele with product of 155 bp; a pair of forward outer primer and a reverse inner primer (NUD-3F1 and NUD-WTR) for detecting normal allele with product of 117 bp. For the NUDT15 c.415C > T genotyping, three product bands of 233 bp (common amplicon), 117 bp (wild-type/G allele-specific amplicon), and 155 bp (variant/A allele-specific amplicon) were expected for heterozygous variant; while homozygous variant has two bands at 233 and 117 bp, and two bands of 233 and 155 bp were expected for wild-type. Primers for amplification are listed in ►Table 1.
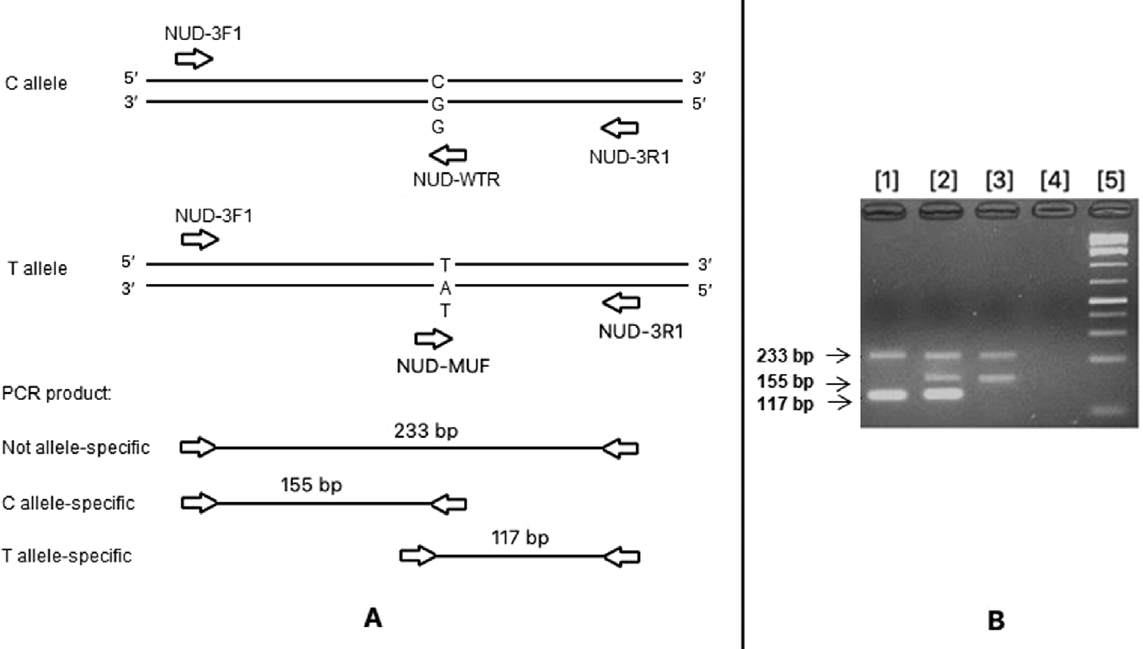
- Genotyping of NUDT15 c.415C > T variant. (A) Schematic illustration of primer design for the tetra-primer amplification refractory mutation system (ARMS) polymerase chain reaction (PCR). (B) Tetra-primer ARMS PCR for detecting c.415C > T variant. Lane 1, sample of patient carrying homozygous variant (T/T); lane 2, sample of patient carrying heterozygous variant (C/T); lane 3, sample of patient without variant (C/C); lane 4, distilled water; lane 5, GeneRuler 1 kp Plus DNA Ladder (Thermo Fisher).
| Primer sequence (5′ – 3′) | Final concentration (µM) | Tm (°C) | Expected product size (bp) | |
|---|---|---|---|---|
| c.55_56insGAGTCG genotype | ||||
| NUD-insF | CTATGACGGCCAGCGCAC | 0.50 | 59°C | Common band: 75 Extra band: 81 |
| NUD-insR | GCTTGCAGCTGGTCACCA | 0.50 | ||
| c.52G > A genotype | ||||
| NUD-1F1 | AGTGAGCGCGTCACTTCCTG | 0.47 | 59°C | Common band: 176 A allele: 120 G allele: 76 |
| NUD-52mR | TTGCAGCTGGTCACCACAAT | 0.33 | ||
| NUD-52wtF | CAGGAGTCGGAGTCGGAG | 0.33 | ||
| NUD-1R2 | AACTGCCAGCTCCAACCG | 0.47 | ||
| c.415C > T genotype | ||||
| NUD-3F1 | TAGGTTAGCTTACCCAAATA | 0.47 | 54°C | Common band: 233 C allele: 155 T allele: 117 |
| NUD-MUF | CCAGCTTTTCTGGGGACCGT | 0.33 | ||
| NUD-WTR | CCTTGTTCTTTTAAACAACG | 0.33 | ||
| NUD-3R1 | CAAATCTTCTCGGCCACCTA | 0.47 | ||
Abbreviations: bp, base pair; PCR, polymerase chain reaction.
Tetra-Primer ARMS PCR
Titration of primer concentrations is essential in tetra-primer ARMS PCR to improve the amplification efficiency of allele-specific products.[11] Tetra-primer ARMS PCR mix for NUDT15 genotyping was prepared as follows: each 15 µL PCR reaction contained 20 ng purified genomic DNA, 0.5 U Takara HS Taq polymerase (Takara Bio, Japan), NUDT15 genotyping primer mix (final concentration of each primer listed in ►Table 1), 0.2 mM dNTP, 1× PCR buffer, with the remaining volume added up to 15 µL by distilled water.
Thermocycling was performed on Eppendorf Mastercycler EP 384 using a three-step PCR program as follows: initial denaturation at 98°C for 3 minutes, followed by 45 cycles of denaturation at 98°C for 10 seconds, annealing at Tm°C for 20 seconds (the annealing temperature of primer sets was listed in ►Table 1), extension at 72°C for 50 seconds, and final extension at 72°C for 2 minutes. Gradient PCR was carried out to determine the best annealing temperature for each primer set.
Two microliters of PCR product from each reaction were electrophoresed in 2.5% agarose gel in 0.5× tris-borate-EDTA buffer at 100 volt for 30 minutes, stained with Gelred (Biotum, United States), and visualized under ultraviolet of WSE-5400 Printgraph Classic (Atto, Japan).
Validation of the Assay
To evaluate the efficiency and accuracy of the assay, the results obtained by tetra-primer ARMS PCR were compared with results from Sanger sequencing analyses.
Results
Sanger Sequencing
From July 2020 to February 2021, 200 ALL patients (aged from 31 months to 14 years) were analyzed for NUDT15 variants using Sanger sequencing. As a result, there were 4 samples with c.52G > A variant on exon 1 (heterozygous only), 11 samples with c.55_56insGAGTCG variant on exon 1 (heterozygous only), 28 samples with c.415C > T variant on exon 3 (including 2 homozygous and 26 heterozygous variants), and 13 samples with compound heterozygous for c.415C > T and c.55_56insGAGTCG variants.
Genotypes of NUDT15 c.55_56insGAGTCG Variant by Conventional PCR
Due to the GGAGTC repeat sequence at the variant region, we were unable to design primers suitable for tetra-primer ARMS PCR for this variant. The conventional PCR was used instead to distinguish wild-type and variant alleles by differences in size on electrophoresis. The reaction was performed with a pair of primers, NUD-insF and NUD-insR. This primer pair had an annealing temperature of 59°C and the final concentration of each primer was 0.50 µM.
Out of 200 patient samples, 24 samples had two bands for heterozygous variant (one band of 75 bp and one band of 81 bp), and 176 samples had one band of 75 bp with normal status (►Fig. 1).
Genotypes of NUDT15 c.52G > A Variant by Tetra-Primer ARMS PCR
Optimize the reaction's temperature: The annealing temperature of NUD-1F1 and NUD-1R2 was found to be 60°C. Next, we continued to investigate the annealing temperature of NUD-1F1 and NUD-52mR as well as NUD-52wtF and NUD-1R2 on a thermal cycler in other reactions with a gradient temperature ranging from 52°C to 60°C (change per reaction was 1°C), and optimum annealing temperature was finalized to be 59°C to produce sharp and clear bands in a single reaction (►Fig. 2B).
Optimize the reaction's concentration of primers: The tetra-primer ARMS PCR was optimized by using three different outer and inner primer concentration ratios of 2:1, 1.5:1, and 1:1 (two inner primers have the same concentration, and two outer primers have the same concentration). We found that the ratio 1.5:1 is suitable for separating PCR fragments from a heterozygote; however, the bands were still blur and unqualified. Then, we finally modified the concentration ratio of 1.4:1 which produced sharp and clear bands. Accordingly, for the c.52G > A variant detection, the final concentration of outer primers was 0.47 µM and of inner primers was 0.33 µM. Compatible with Sanger sequencing results, all 4 samples with heterozygous variant had three bands of 176, 120, and 76 bp. The 196 remaining samples with normal status had two bands of 176 and 76 bp (►Fig. 2B). Homozygous variant was not found in the group of patients participating in this study, indicating that homozygous variant is extremely rare in our population.
Genotypes of NUDT15 c.415C > T Variant by Tetra-primer ARMS PCR
Optimize the reaction's temperature: The annealing temperatures of NUD-3F1 and NUD-3R1 were found to be 60°C. For other primer pairs (NUD-3F1 and NUD-WTR; NUD-MUF and NUD-3R1), to identify the optimal annealing temperature we ran PCR reactions with a gradient temperature from 52°C to 60°C. As a result, 54°C was chosen as the best annealing temperature.
Optimize the reaction's concentration primers: The primer concentration for detecting variant c.415C > T was chosen in the same way for variant c.52G > A. The final concentration of outer primers was 0.47 µM and that of inner primers was 0.33 µM.
Out of 200 patient samples, 2 samples had two bands of 233 and 117 bp, corresponding to a homozygous variant, while the 39 remaining samples had three bands (233, 155, and 117 bp) corresponding to a heterozygous status. All 159 wild-type samples had two bands of 233 and 155 bp (►Fig. 3B).
Validation of PCR Procedure by Direct Sequencing
For each variant analyzed, the electrophoresis results of the PCR products were completely consistent with the Sanger sequencing results. The PCR product did not detect an extra band of variant in any of the wild-type samples, indicating that PCR has 100% specificity compared with Sanger sequencing. On the other hand, PCR fully detected all 3 variants in the heterozygous form as well as c.415G > A variant in homozygous form, suggesting that PCR has 100% sensitivity when compared with Sanger sequencing.
Discussions
The NUDT15 variation is important in determining drug toxicity for treatment of ALL in Asian as well as Vietnamese patients. We have recently shown that the NUDT15 genetic variants are the most common polymorphisms and significantly affect 6-MP-induced toxicity in a cohort of 70 Vietnamese pediatric ALL patients.[12] Although Sanger sequencing is the gold standard for detecting variants, a simple PCR method is more useful in clinical applications. In this study, we were successful in developing simple PCR procedure for detecting the three most prevalent NUDT15 variants (c.52G > A, c.55_56insGAGTCG, and c.415C > T) in Vietnamese patients.
Several studies have established PCR for the detection of c.52G > A and c.415C > T variants of NUDT15,[13,14] but to our knowledge, this is the first study using PCR-only technique to detect all three variants. Moreover, for genotyping the NUDT15 Grover et al utilized PCR-restriction fragment length polymorphism,[13] while Ho et al established a tetra-primer PCR without a mismatch at third position from the 3′-end of the designed primers.[14] The PCR method is an effective technique that incorporates amplification and genotyping into a single step. In addition, this method is especially suitable for basic molecular laboratories with limited equipment. This simple PCR procedure can be performed with a thermal cycler and electrophoresis system, in combination with PCR reagents and carefully designed oligonucleotide primers. Moreover, the cost of this procedure is much less expensive compared with Sanger sequencing for detecting common NUDT15 variants (5.8 USD vs. 12.7 USD in Vietnam). However, it should be kept in mind that the PCR-only technique cannot identify any other variants in NUDT15 besides the three variants described here.
In this study, we used ALL patient samples to develop the PCR procedure, to detect NUDT15 gene variants for thiopurine treatment. However, thiopurine is indicated not only for ALL; it is also used for maintenance therapy in inflammatory bowel disease including maintenance of remission in Crohn's disease and ulcerative colitis patients who are steroid dependent and refractory.[15] As a result, the PCR procedure we developed can be used to detect NUDT15 variants for thiopurine monitoring in a variety of disorders.
Conclusion
In basic molecular laboratories with limited equipment, our PCR procedure is suitable for replacing Sanger sequencing to detect the NUDT15 variants in clinical setting. This can help to determine effective therapies and appropriate dosing of thiopurines.
Conflict of Interest
None delcared.
Funding
This study was financially supported in part by University of Medicine and Pharmacy at Ho Chi Minh City.
References
- Acute Lymphoblastic Leukemia in Adults. The EBMT Handbook; 2019:531-538.
- [CrossRef] [PubMed] [Google Scholar]
- The relationship of genetics, nursing practice, and informatics tools in 6-mercaptopurine dosing in pediatric oncology [Formula: see text] J Pediatr Oncol Nurs. 2017;34(05):342-346.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical pharmacogenetics implementation consortium guideline for thiopurine dosing based on TPMT and NUDT 15 genotypes: 2018 update. Clin Pharmacol Ther. 2019;105(05):1095-1105.
- [CrossRef] [PubMed] [Google Scholar]
- The promise of pharmacogenomics in reducing toxicity during acute lymphoblastic leukemia maintenance treatment. Genomics Proteomics Bioinformatics. 2017;15(02):82-93.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of TPMT, NUDT15, and ITPA genetic variants on 6-mercaptopurine toxicity for pediatric patients with acute lymphoblastic leukemia in Yunnan of China. Front Pediatr. 2021;9 719803
- [CrossRef] [PubMed] [Google Scholar]
- Predictive role of NUDT15 variants on thiopurine-induced myelotoxicity in Asian inflammatory bowel disease patients. Pharmacogenomics. 2018;19(01):31-43.
- [CrossRef] [PubMed] [Google Scholar]
- Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J Clin Oncol. 2015;33(11):1235-1242.
- [CrossRef] [PubMed] [Google Scholar]
- NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet. 2016;48(04):367-373.
- [CrossRef] [PubMed] [Google Scholar]
- Guidelines for the tetra-primer ARMS-PCR technique development. Mol Biotechnol. 2014;56(07):599-608.
- [CrossRef] [PubMed] [Google Scholar]
- Development of tetra-primer amplification refractory mutation system (ARMS) PCR for detection of CHRNA3 rs8040868. Indones Biomed J. 2021;13:192-200.
- [CrossRef] [Google Scholar]
- Designing, optimization and validation of tetra-primer ARMS PCR protocol for genotyping mutations in caprine Fec genes. Meta Gene. 2014;2:439-449.
- [CrossRef] [PubMed] [Google Scholar]
- The effects of NUDT15 and TPMT variants on mercaptopurine treatment in Vietnamese pediatric acute lymphoblastic leukemia patients. Pediatr Hematol Oncol. 2022;39(06):561-570.
- [CrossRef] [PubMed] [Google Scholar]
- TPMT and NUDT15 polymorphisms in thiopurine induced leucopenia in inflammatory bowel disease: a prospective study from India. BMC Gastroenterol. 2021;21(01):327.
- [CrossRef] [PubMed] [Google Scholar]
- Novel tetra-primer ARMS-PCR assays for thiopurine intolerance susceptibility mutations NUDT15 c.415C>T and TPMT c.719A>G (TPMT* 3C) in East Asians. Genes (Basel). 2017;8(10):285-293.
- [CrossRef] [PubMed] [Google Scholar]
- Monitoring thiopurine metabolites in inflammatory bowel disease. Frontline Gastroenterol. 2016;7(04):301-307.
- [CrossRef] [PubMed] [Google Scholar]





