Translate this page into:
A Study on the Serum γ-Glutamyltranspeptidase and Plasma Osteopontin in Alcoholic Liver Disease
Address for correspondence: Naveen Singh, MSc, Department of Biochemistry, Faculty of Medicine and Health Sciences, SGT University, Gurugram, Haryana, 122505, India (e-mail: singh.naveen95@gmail.com).
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background
Alcoholic liver disease (ALD) is a major source of alcohol-related morbidity and mortality. Heavy drinkers and alcoholics may progress from fatty liver to alcoholic hepatitis to cirrhosis. The enzyme γ-glutamyltranspeptidase (GGT) is a membrane-bound glycoprotein which catalyzes the transfer of the γ-glutamyl group from γ-glutamyl peptides to other peptides, amino acids, and water. Serum GGT activity mainly attributed to hepatobiliary system and thus is an important marker of ALD. Hence the present study is conducted to estimate and correlate the levels of GGT and osteopontin (OPN) in ALD.
Aims and Objectives
The objective of this study is to estimate and correlate the levels of GGT and OPN in ALD.
Materials and Methods
Sixty clinically diagnosed cases of ALD and sixty age- and gender-matched healthy controls were recruited for the study. Blood samples were collected from them and serum aspartate aminotransferase, serum alanine transaminases (ALTs), serum ALP levels, and plasma OPN levels were measured. Estimation of serum aspartate transaminases (AST), ALTs, and alkaline phosphatase (ALP) was assayed by standard photometric methods in autoanalyzer ERBA-XL (EM-200) using commercially available kits. OPN was estimated by using commercial kit based on enzyme-linked immunosorbent assay.
Results
The parameters of the liver function tests such as AST, ALT, and ALP were significantly increased in patients with ALD (p < 0.001) when compared with the healthy control subjects. In the present study, significantly increased levels of γ-glutamyl transferases and OPN were found in patients with ALD (p < 0.001) when compared with the control subjects. OPN showed significant positive correlations with AST (r = 0.76, p < 0.001), ALT (r = 0.64, p < 0.001), ALP (r = 0.68, p < 0.001), and GGT (r = 0.61, p < 0.001).
Conclusion
The present study focuses on the role of GGT and OPN that are sensitive indicators of liver cell injury and are most helpful in recognizing hepatocellular diseases such as ALD, hepatitis, and liver cirrhosis. Hence, the pattern of the GGT and OPN levels elevation can be helpful diagnostically.
Keywords
GGT
osteopontin
alcoholic liver disease
AST
ALT
ALP
Introduction
Alcohol remains a major cause of liver disease worldwide. The amount of alcohol ingested (independent of the form in which it is ingested) is the most important risk factor for the development of alcoholic liver disease (ALD).[1] Alcoholism remains the most important and apparent cause of cirrhosis, especially in the Western world. Intake of 80 g of alcohol per day has been defined as “hazardous drinking” and consumption of amounts in excess significantly increases the risk of developing cirrhosis.[2] ALD is a major source of alcohol-related morbidity and mortality. Heavy drinkers and alcoholics may progress from fatty liver to alcoholic hepatitis (AH) to cirrhosis, and it is estimated that 10 to 15% of alcoholics will develop cirrhosis.[3] The risk of developing cirrhosis increases with an average alcohol ingestion of > 60 to 80 g/d in men and > 20 g/d for ≥ 10 years in women (A unit of alcohol contains 8 g of ethanol).[4,5] A threshold for developing ALD is variable but begins at 30 g/d of ethanol. However, there is no clear linear relationship between dose and liver damage.[6] Previous studies have reported that in 90% of individuals who had continued alcohol use (> 40 g/d), there was an increased risk of progression to cirrhosis in 30%, and fibrosis or cirrhosis in 37%.[7,8] The aim of the present study is to determine the correlation of GGT with osteopontin (OPN) and liver enzymes in the diagnosis of ALD. The enzyme γ-glutamyltranspeptidase (GGT) is a membrane bound glycoprotein which catalyzes the transfer of the γ-glutamyl group from γ-glutamyl peptides to other peptides, amino acids, and water.[9] It occurs predominantly as a membrane bound enzyme in significant amounts only in the kidney, pancreas, liver, spleen, and small intestine. GGT is a microsomal enzyme present in hepatocytes and biliary epithelial cells, renal tubules, pancreas, and intestine.[10] It is also present in cell membrane performing transport of peptides into the cell across the cell membrane and involved in glutathione metabolism. Serum GGT activity mainly attributed to hepatobiliary system even though it is found in more concentration in renal tissue.[11] Raised serum activity of the enzyme has been reported in alcoholism, various forms of liver diseases including primary and secondary hepatic tumors, diabetes mellitus, cardiovascular disease, renal neoplasms, and the nephrotic syndrome. A recent study of its value in liver disease has shown that serum GGT activity correlates closely with the activity of alkaline phosphatase (ALP) and 5-nucleotidase, and that compared with these two other enzymes, GGT is a more sensitive index of biliary tract disease.[10] OPN first described as a phosphoprotein was secreted by a transformed cell line in 1979. Several years later, the bone-specific sialoprotein was cloned as a matrix protein and termed as OPN.[12] OPN is located on chromosome 4 region 22 (4q22.1) in humans and is composed of approximately 300 amino acids (314 in human, but 297 in mouse). The multifunctionality of OPN is due to varied post-translational modifications such as phosphorylation, sulfation, glycosylation, and proteolytic cleavage. OPN contains an arginine-glycine-aspartate domain, which binds with high affinity to integrins such as αvβ1, αvβ3, αvβ5, αvβ6, α8β1, and α5β1.[13] OPN has two isoforms, a secreted form of OPN (sOPN) and an intracellular form of OPN (iOPN). sOPN staining had perinuclear distribution which appeared in Golgi, and iOPN staining had perimembrane distribution.[14-16] Hepatic expression and plasma levels of OPN are markedly elevated in patients with AH, alcoholic cirrhosis, and end stage ALD.[17] OPN is progressively increased in liver fibrosis and is associated with the stage of fibrosis.[9] OPN may play a protective role and its deficiency facilitates the development of AH, neutrophilic inflammation, and high mortality rate. It is established that OPN could bind to gut-derived lipopolysaccharides and prevent macrophage activation, reactive oxygen species, nitrogen species generation, and tumor necrosis factor-α (TNFα).[18]
Materials and Methods
The present hospital-based cross-sectional case–control study was performed in the Department of Biochemistry and Medicine, Faculty of Medicine & Health Sciences, SGT Medical College, Hospital & Research Institute (SGT University), Gurugram, Haryana. Sixty clinically diagnosed patients of ALD in the age group of 20 to 60 years attending Medicine OPD of SGT Hospital, Budhera, Gurugram were included as the cases. Cases were defined based on medical history, physical examination, and laboratory investigations. Sixty (60) age- and gender-matched healthy volunteers from general population were taken as controls.
After explaining, the purpose and details of the study to all the subjects of both the groups, a written and informed consent was taken. Ethical clearance was obtained from the Institutional Ethical Committee before starting the collection of samples. Patients with alcohol intake ≥100 g/d, duration of alcohol intake >8 years and serum aspartate transaminases (AST) levels two to six times raised as compared with alanine transaminases (ALT) were included as cases. Patients with viral hepatitis B and C, autoimmune hepatitis, drug-induced hepatitis, copper and iron storage disease (Wilson disease, hemochromatosis), chronic smoking, steroids, antihypertensive drugs, hypoglycemic drugs or hormone replacement therapy, concomitant inflammatory disorders, and renal disorders (was ruled out on the basis of kidney function test) were excluded from the study.
A total of 5 mL of venous blood was collected after 12 to 14 hours of fasting taking all aseptic precautions. Out of 5 mL, 3 mL was collected in plain vial and serum was separated by centrifuging at 3,000 rpm for 10 to 15 minutes and 2 mL blood was collected in an ethylenediaminetetraacetic acid (EDTA) containing vial for the estimation of OPN. Liver function tests of the subjects were estimated immediately, and one aliquot was preserved at 20°C for the estimation of OPN within 1 month of collection of samples. Internal quality control for all the tests was performed using control materials obtained from ERBA, Germany.
Estimation of AST, ALT, ALP, and γ-glutamyl transferases (GGT) was assayed by standard photometric methods in autoanalyzer ERBA-XL (EM-200) using commercially available kits.
GGT was estimated by The International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) method with L-γ-glutamyl-3-carboxy-4-nitroanilide as substrate. GGT present in the sample catalyzes the transfer of the glutamyl group from the substrate γ-glutamyl-3-carboxy-4-nitroanilide to glycylglycine forming glutamyl-glycyl-glycine and 5-amino-2-nitrobenzoate. The rate of formation of 5-amino-2-nitrobenzoate is proportional to the activity of GGT present in the sample and can be measured kinetically at 400 to 420 nm. Reference range for males: < 55 U/L and for females: < 38 U/L.
Human OPN immunoassay (Quantikine ELISA, United States of America. Catalog No. DOST00) employs the quantitative sandwich enzyme immunoassay technique. A monoclonal antibody specific for human OPN has been precoated onto a microplate. Standards and samples are pipetted into the wells and any OPN present was bound by the immobilized antibody. After washing away any unbound substances, an enzyme-linked polyclonal antibody specific for human OPN was added to the wells. Following a wash to remove any unbound antibody-enzyme reagent, a substrate solution was added to the wells and color develops in proportion to the amount of OPN bound in the initial step. The color development was stopped and the intensity of the color was measured. Reference range is 53.4 ng/mL to 195 ng/mL.
Serum aspartate aminotransferase (AST) was estimated by IFCC recommended method without pyridoxal phosphate. AST present in the sample catalyzes the transfer of the amino group from L-aspirate to 2-oxoglutarate forming oxaloacetate and L-glutamate. Oxaloacetate in the presence of NADH and malate dehydrogenase is reduced to L-malate. In this reaction, NADH is oxidized NADH+. The reaction is monitored by measuring the rate of decrease in absorbance at 340 nm due to oxidation of NADH to NAD+. Addition of lactate dehydrogenase (LDH) to the reagent is necessary to achieve rapid and complete reduction of endogenous pyruvate so that it is does not interfere with the assay. Reference range for male is up to 35 U/L and for female is up to 31 U/L.
Serum ALT was estimated by IFCC recommended method without pyridoxal phosphate. The amino group is enzymatically transferred by ALT present in the sample from alanine to the carbon atom of 2-oxoglutrate yielding pyruvate and L-glutamate. Pyruvate is reduced to lactate by LDH present in the reagent with the simultaneous oxidation of NADH to NAD+. The reaction was monitored by measuring the rate of decrease in absorbance at 340 nm due to the oxidation of NADH. Endogenous sample pyruvate was rapidly and completely reduced by LDH during initial incubation period to avoid interference during assay. Reference range in males is up to 45 U/L and in females is up to 34 U/L.
ALP was estimated by 2-amino-2-methyl-1-propanol method. This method utilizes 4-nitrophenyl phosphate as the substrate. At the pH of the reaction, 4-nitrophenol has an intense yellow color. The reagent also contains a metal ion buffer system to ensure that optimal concentrations of zinc and magnesium are maintained. The metal ion buffer can also chelate other potentially inhibitory ions which may be present. The reaction is monitored by measuring the rate of increase in absorbance at 415 nm which is proportional to the activity of ALP in the serum. Reference range in male is 53 to 128 U/L for 20 to 59 years and 56 to 119 U/L for ≥ 60 years. For females between 20 and 59 years it is 42 to 98 U/L and for females ≥ 60 years it is 53 to 141 U/L.
Statistical Analysis
The data recorded was entered in a spreadsheet and then statistical analyses were performed by using Statistical Package for the Social Sciences (SPSS) Version 21.0. Continuous variables were summarized in the form of means and standard deviations. Graphical data was presented by bar diagrams. Student's independent t-test was employed for comparing continuous variables. Pearson's correlations coefficient was applied to study the association of OPN with liver enzymes of ALD patients. The p-value (p < 0.05) was considered statistically significant for all the parameters.
Results
The mean age of the ALD patients was 53.06 ± 4.38 years and for the healthy controls was 50.76 ± 5.89 years (►Table 1). In our study, 93% cases of ALD were males (n = 56) and 7% were females (n = 4), whereas 92% of the controls were males (n = 55) and 7% were females (n = 5) (►Figs. 1 and 2).
- Distribution of ALD cases according to gender. ALD, alcoholic liver disease.
- Distribution of controls according to gender.
| Parameters | Cases (n = 60) | Controls (n = 60) | |
|---|---|---|---|
| Sex | Male | 56 | 55 |
| Female | 04 | 05 | |
| Age (years) | 53.06 ± 4.38 | 50.76 ± 5.89 |
The parameters of the liver function tests such as AST, ALT, and ALP were significantly increased in the patients with ALD (p < 0.001) when compared with the healthy control subjects (►Table 2).
| Parameters | Cases (Mean ± SD) | Controls (Mean ± SD) | t-Value | p-Value |
|---|---|---|---|---|
| GGT (U/L) | 46.14 ± 9.06 | 28.96 ± 9.28 | 10.25 | 0.001a |
| OPN (ng/mL) | 97.43 ± 31.59 | 34.33 ± 11.28 | 14.56 | 0.001a |
Abbreviations: ALD, alcoholic liver disease; GGT, γ-glutamyltranspeptidase; OPN, osteopontin; SD, standard deviation.
a p < 0.001
►Table 3 showed significant increase in the parameters of liver function tests (AST, ALT, ALP, and AST/ALT ratio) of ALD patients when compared with the controls and was statistically significant at the level of p < 0.001 (►Figs. 3-5).
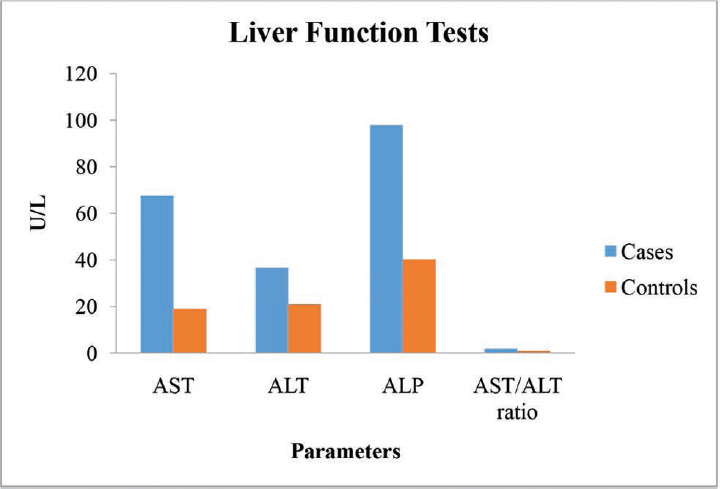
- Graph showing liver function tests of the ALD patients and the controls. ALD, alcoholic liver disease.
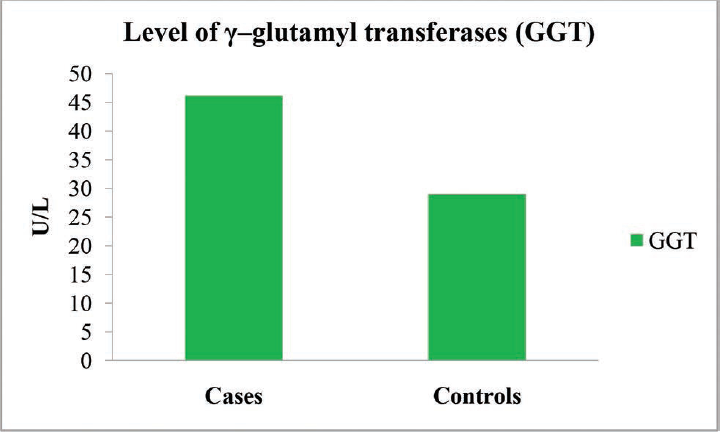
- Graph showing the level of γ–glutamyl transferases (GGT) of the ALD patients and the controls. ALD, alcoholic liver disease.
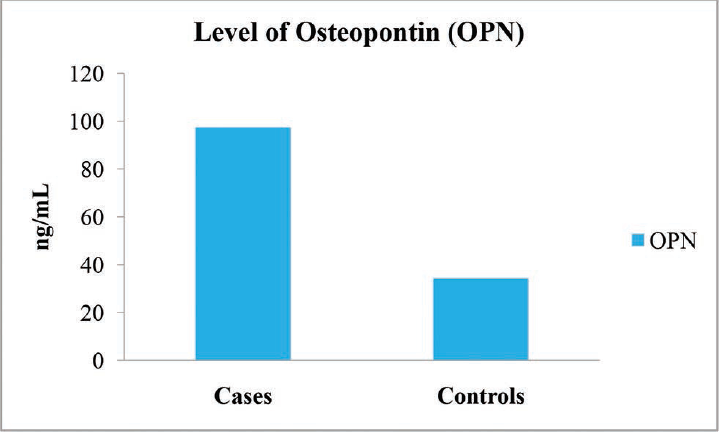
- Graph showing the level of osteopontin (OPN) of the ALD patients and the controls. ALD, alcoholic liver disease.
| Parameters | Cases (Mean ± SD) | Controls (Mean ± SD) | t-Value | p-Value |
|---|---|---|---|---|
| AST (U/L) | 67.48 ± 9.43 | 18.86 ± 3.79 | 37.04 | 0.001a |
| ALT (U/L) | 36.58 ± 5.81 | 20.98 ± 5.02 | 15.72 | 0.001a |
| ALP (U/L) | 97.90 ± 29.45 | 40.26 ± 8.01 | 14.62 | 0.001a |
| AST/ALT ratio | 1.86 ± 0.27 | 0.93 ± 0.24 | 19.73 | 0.001a |
Abbreviations: ALD, alcoholic liver disease; ALP, alkaline phosphatase; ALT, alanine transaminases; AST, aspartate aminotransferase; SD, standard deviation.
a p < 0.001
In the present study, significantly increased levels of GGT and OPN were found in the patients with ALD (p < 0.001) when compared with the control subjects (►Table 2).
►Table 2 showed significant increase in the levels of markers of ALD (GGT and OPN) of ALD patients when compared with the controls and was statistically significant at the level of p < 0.001.
In the present study significant correlations between the level of OPN and the liver enzymes (AST, ALT and ALP) were found (►Table 4). OPN showed significant positive correlations with AST (r = 0.76, p < 0.001), ALT (r = 0.64, p < 0.001), ALP (r = 0.68, p < 0.001), and GGT (r = 0.61, p < 0.001) (►Figs. 6–9).
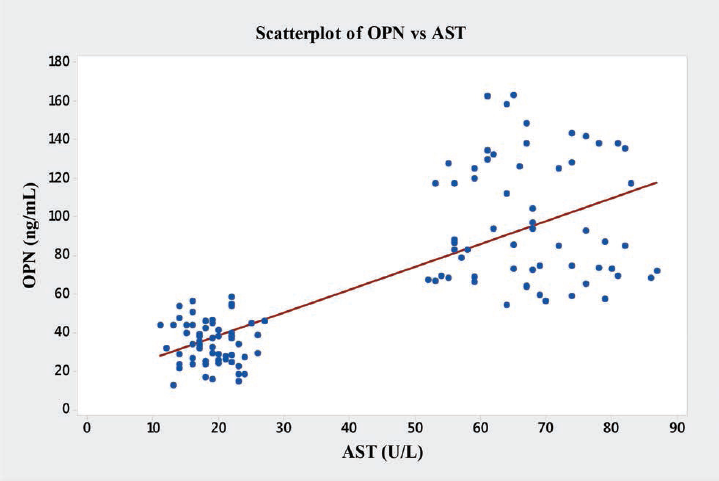
- Graph showing Pearson's correlations of OPN and AST of the subjects. AST, aspartate aminotransferase; OPN, osteopontin.
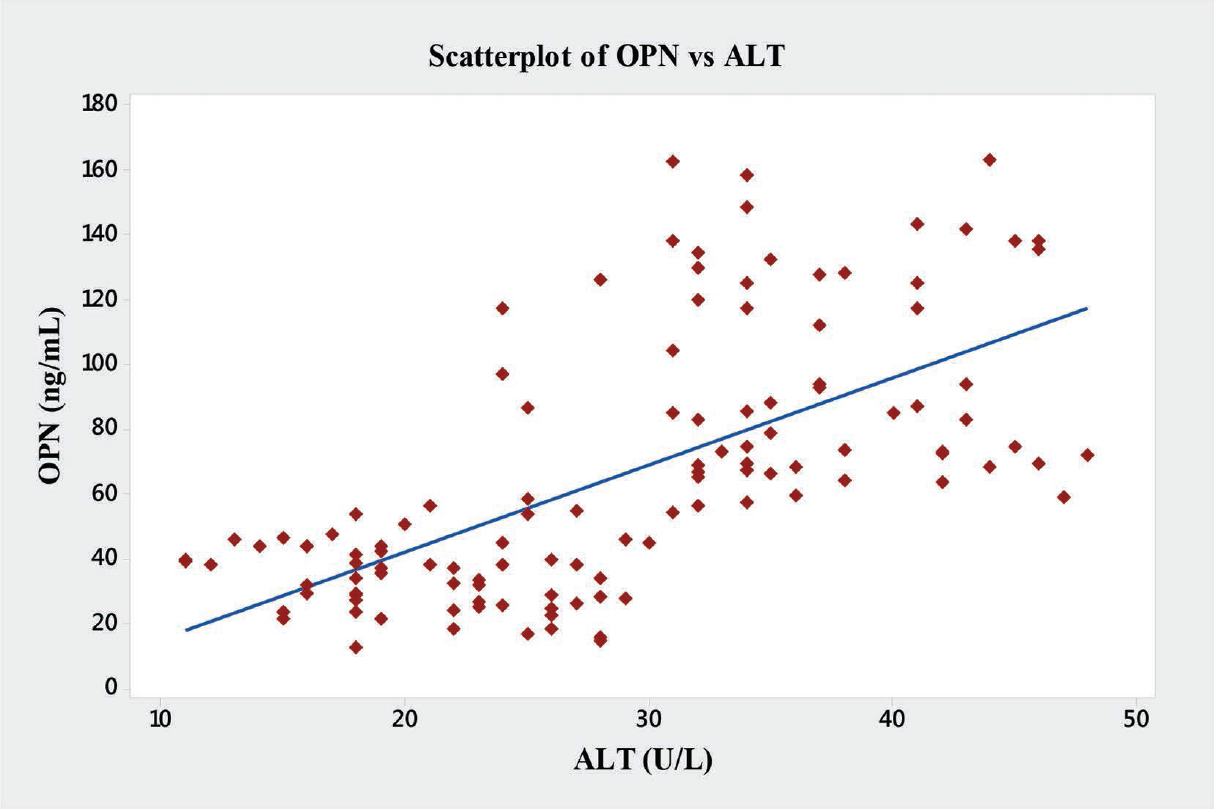
- Graph showing Pearson's correlations of OPN and ALT of the subjects. ALT, alanine transaminases; OPN, osteopontin.
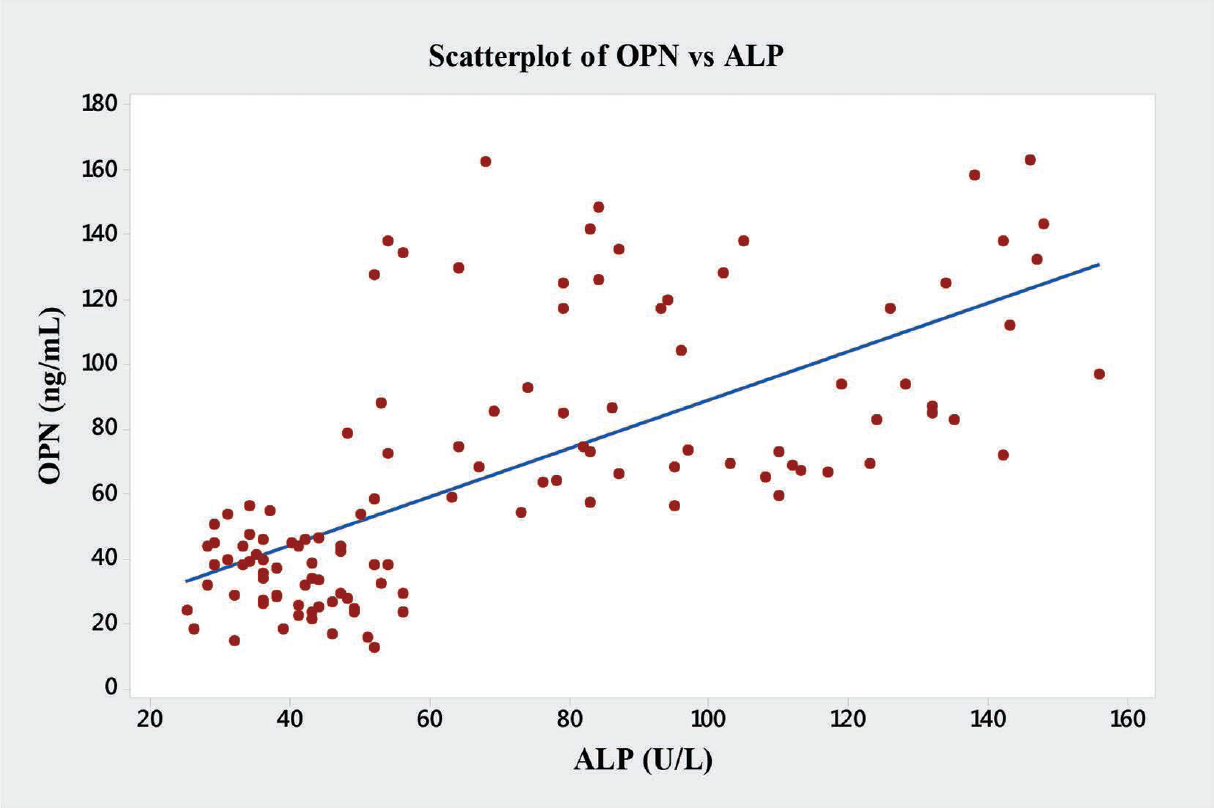
- Graph showing Pearson's correlations of OPN and ALP of the subjects. ALP, alkaline phosphatase; OPN, osteopontin.
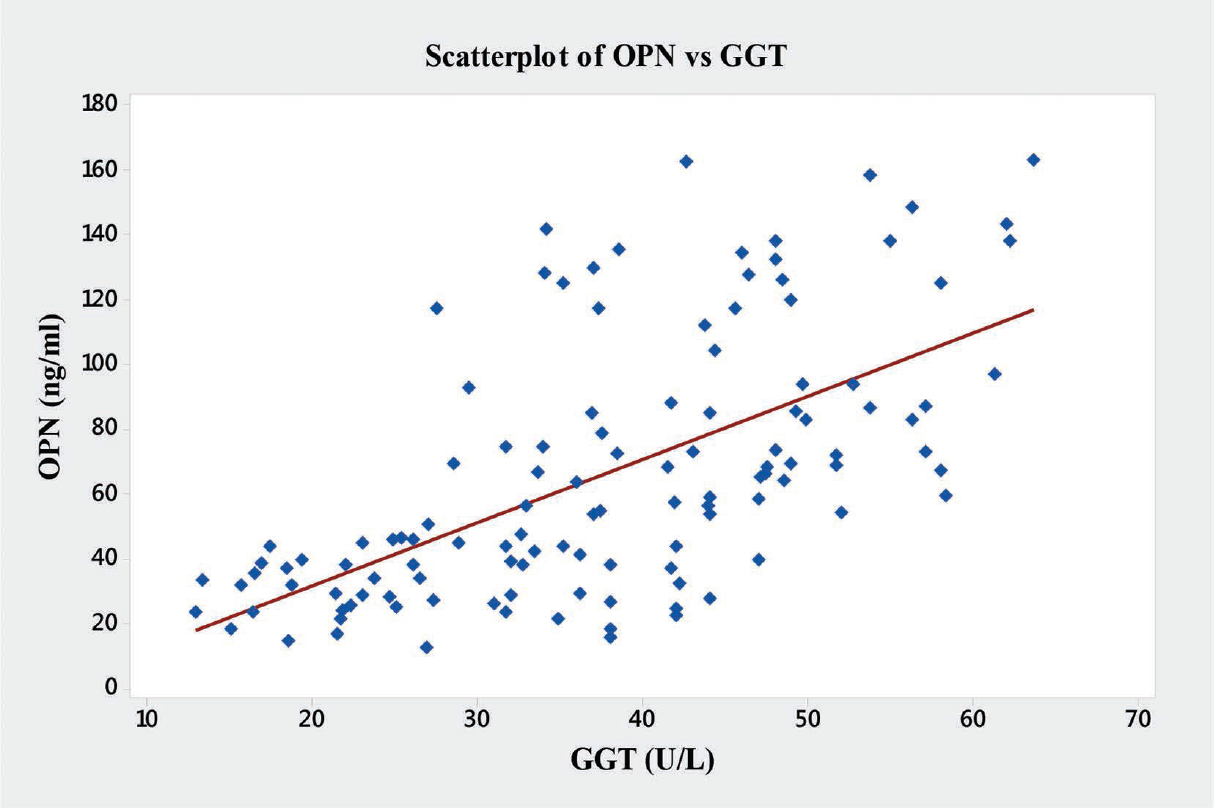
- Graph showing Pearson's correlations of OPN and GGT of the subjects. GGT, γ-glutamyltranspeptidase; OPN, osteopontin.
| Variables | Osteopontin R | p-Value |
|---|---|---|
| AST | 0.76 | 0.001a |
| ALT | 0.64 | 0.001a |
| ALP | 0.68 | 0.001a |
| GGT | 0.61 | 0.001a |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine transaminases; AST, aspartate aminotransferase; GGT, γ-glutamyltranspeptidase.
a Correlation is significant at p < 0.05.
b p < 0.001
Discussion
In our study, we found that liver enzymes such as AST, ALT, ALP, and GGT were significantly increased in the patients with ALD (p < 0.001) when compared with the healthy control subjects. Similar findings were observed by Cuschieri and Baker,[10] Konttinen et al,[19] Matloff et al,[20] Moussavian et al,[21] Das and Vasudevan,[22] Maithreyi et al,[23] Mirunalini et al,[24] Jang et al,[25] Al-Jumaily,[26] Hyder et al,[27] Gayathri and Vasantha,[28] and Brandl et al.[29] However, our findings are contradictory to the study conducted by Hourigan and Bowling[30] who found normal levels of serum AST and ALT in patients with alcoholic cirrhosis. Whereas, in the study conducted by Seth et al[31]there was no significant difference in serum ALT level between the cases and controls (p = 0.21).
In addition, in the study conducted by Teschke et al[32] and Benerji et al[33], serum ALP in patients with ALD was nonsignificant (p < 0.10) when compared with the controls. Similarly, Agarwal et al34 found a significantly lower level of ALP (5–6%, p < 0.01) in alcohol consumers compared with the nonconsumers.
In our study, we found significant increase in the level of OPN in the patients with ALD (p < 0.001) when compared with the control subjects. This is inconsistent with the findings of Wen et al,[13] Arai et al,[35] Zhao et al,[36] Patouraux et al,[37] Seth et al,[31] and Fouad et al.[38]
In the present study, we have also observed novel and significant associations between the level of OPN and the liver enzymes (AST, ALT, ALP, and GGT). OPN showed significant positive correlations with AST (r = 0.76, p < 0.001), ALT (r = 0.64, p < 0.001), ALP (r = 0.68, p < 0.001), and GGT (r = 0.61, p < 0.001) (►Fig. 9). We report here for the first time the significant correlation of plasma OPN level with liver enzymes in the patients with ALD. However, Moussavian et al reported that, serum levels of GGT correlated significantly with the levels of serum glutamic oxaloacetic transaminase or SGOT (p < 0.001, r = 0.55), serum glutamic pyruvic transaminase or SGPT (p < 0.001, r = 0.45), ALP (p < 0.05, r = 0.49), and serum total bilirubin (p < 0.05, r = 0.53). Further, they concluded that, serum GGT was persistently elevated in alcoholic patients with liver injury.[21] Gogoi et al found significant correlation in the level of serum GGT in alcoholics when compared with the normal subjects.[39] Srungaram et al concluded that, OPN levels correlated with the degree of liver necrosis in acute liver failure and very high levels of OPN were associated with hyperactive acute injury and good outcomes.[40]
Conclusion
The present study focuses on the role of OPN and aminotransferases (transaminases) that are sensitive indicators of liver cell injury and are most helpful in recognizing hepatocellular diseases such as ALD, hepatitis, and liver cirrhosis. Hence, the pattern of the GGT and OPN levels elevation can be helpful diagnostically.
Conflict of Interest
None.
References
- Alcohol consumption and alcoholic liver disease: evidence of a threshold level of effects of ethanol. Alcohol Clin Exp Res. 1993;17(05):1112-1117.
- [CrossRef] [PubMed] [Google Scholar]
- Pathogenesis of alcoholic liver disease: newer mechanisms of injury. Keio J Med. 1999;48(04):184-188.
- [CrossRef] [PubMed] [Google Scholar]
- The epidemiology of alcoholic liver disease. Alcohol Res Health. 2003;27(03):209-219.
- [Google Scholar]
- Histologic spectrum of alcoholic liver disease. Semin Liver Dis. 1986;6(03):221-232.
- [CrossRef] [PubMed] [Google Scholar]
- Morphology of alcoholic liver disease. Clin Liver Dis. 2005;9(01):37-53.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of alcoholic liver disease. Semin Liver Dis. 2004;24(03):217-232.
- [CrossRef] [PubMed] [Google Scholar]
- The Dionysos Study Group. Drinking habits as cofactors of risk for alcohol induced liver damage. Gut. 1997;41(06):845-850.
- [CrossRef] [PubMed] [Google Scholar]
- Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;154(02):299-315.
- [CrossRef] [PubMed] [Google Scholar]
- Liver function tests and their interpretation. Indian J Pediatr. 2007;74(07):663-671.
- [CrossRef] [PubMed] [Google Scholar]
- Gamma-glutamyl-transpeptidase in hepato-biliary disease—value as an enzymatic liver function test. Br J Exp Pathol. 1974;55(02):110-115.
- [Google Scholar]
- Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. (4th). Philadelphia, PA: Elsevier; 2006. p. :604-616.
- [Google Scholar]
- Osteopontin: versatile modulator of liver diseases. Hepatol Res. 2014;44(01):22-30.
- [CrossRef] [PubMed] [Google Scholar]
- Role of osteopontin in liver diseases. Int J Biol Sci. 2016;12(09):1121-1128.
- [CrossRef] [PubMed] [Google Scholar]
- Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc Natl Acad Sci U S A. 1986;83(23):8819-8823.
- [CrossRef] [PubMed] [Google Scholar]
- A biochemical characterization of the binding of osteopontin to integrins alpha v beta 1 and alpha v beta 5. J Biol Chem. 1995;270(44):26232-26238.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of osteopontin as a novel ligand for the integrin alpha8 beta1 and potential roles for this integrin-ligand interaction in kidney morphogenesis. Mol Biol Cell. 1998;9(06):1425-1435.
- [CrossRef] [PubMed] [Google Scholar]
- Appendix: therapeutic drug monitoring and laboratory reference ranges. In: Stephen JM, Maxine AP, eds. Current medical diagnosis and treatment (46th). New York, NY: McGraw Hill; 2007. p. :1767-1775.
- [Google Scholar]
- Transformed mammalian cells secrete specific proteins and phosphoproteins. Cell. 1979;16(04):885-893.
- [CrossRef] [PubMed] [Google Scholar]
- Multiple serum enzyme analyses in chronic alcoholics. Acta Med Scand. 1970;188(04):257-264.
- [CrossRef] [PubMed] [Google Scholar]
- Hepatic transaminase activity in alcoholic liver disease. Gastroe-nterology. 1980;78(06):1389-1392.
- [CrossRef] [PubMed] [Google Scholar]
- Serum gamma-glutamyl transpeptidase and chronic alcoholism. Dig Dis Sci. 1985;30(03):211-214.
- [CrossRef] [PubMed] [Google Scholar]
- Biochemical diagnosis of alcoholism. Indian J Clin Biochem. 2005;20(01):35-42.
- [CrossRef] [Google Scholar]
- Erythrocyte lipid peroxidation and antioxidants in chronic alcoholics with alcoholic liver disease. Asian J Pharm Clin Res. 2010;3:183-185.
- [Google Scholar]
- Curative effect of garlic on alcoholic liver diseased patients. Jordan J Biol Sci. 2010;3(04):147-152.
- [Google Scholar]
- Effects of coffee, smoking, and alcohol on liver function tests: a comprehensive cross-sectional study. BMC Gastroenterol. 2012;12(01):145.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of chronic liver diseases on some biochemical parameters in patients serum. Curr Res J Biol Sci. 2012;4(05):638-642.
- [Google Scholar]
- Comparative levels of ALT, AST, ALP and GGT in liver associated diseases. Eur J Exp Biol. 2013;3(02):280-284.
- [Google Scholar]
- Comparative levels of liver enzymes in patients with various liver disorders. Int J Pharma Bio Sci. 2015;6(04):1099-1102.
- [Google Scholar]
- Dysregulation of serum bile acids and FGF19 in alcoholic hepatitis. J Hepatol. 2018;69(02):396-405.
- [CrossRef] [PubMed] [Google Scholar]
- Alcoholic liver disease: a clinical series in an Australian private practice. J Gastroenterol Hepatol. 2001;16(10):1138-1143.
- [CrossRef] [PubMed] [Google Scholar]
- Osteopontin is an important mediator of alcoholic liver disease via hepatic stellate cell activation. World J Gastroenterol. 2014;20(36):13088-13104.
- [CrossRef] [PubMed] [Google Scholar]
- Hepatic gamma-glutamyltransferase activity in alcoholic fatty liver: comparison with other liver enzymes in man and rats. Gut. 1983;24(07):625-630.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative study of ALT, AST, GGT & uric acid levels in liver diseases. IOSR-JDMS. 2013;7(05):72-75.
- [CrossRef] [Google Scholar]
- Assessing alcohol intake & its dose dependent effects on liver enzymes by 24-h recall and questionnaire using NHANES 2001-2010 data. Nutrition Journal 2016:15-62.
- [CrossRef] [PubMed] [Google Scholar]
- Serum osteopontin levels in patients with acute liver dysfunction. Scand J Gastroenterol. 2006;41(01):102-110.
- [CrossRef] [PubMed] [Google Scholar]
- Elevated plasma osteopontin level is predictive of cirrhosis in patients with hepatitis B infection. Int J Clin Pract. 2008;62(07):1056-1062.
- [CrossRef] [PubMed] [Google Scholar]
- The osteopontin level in liver, adipose tissue and serum is correlated with fibrosis in patients with alcoholic liver disease. PLoS One. 2012;7(04):e35612.
- [CrossRef] [PubMed] [Google Scholar]
- Plasma osteopontin level in chronic liver disease and hepatocellular carcinoma. Hepat Mon. 2015;15(09):e30753.
- [CrossRef] [PubMed] [Google Scholar]
- Study of serum gamma glutamyltransferase as a diagnostic marker in alcoholic hepatitis. J Pharm. 2012;2(04):69-71.
- [CrossRef] [Google Scholar]
- Acute Liver Failure Study Group. Plasma osteopontin in acute liver failure. Cytokine. 2015;73(02):270-276.
- [CrossRef] [PubMed] [Google Scholar]





