Translate this page into:
A study to assess the prevalence and risk factors for Clostridioides difficile infection in patients with inflammatory bowel disease in a tertiary care hospital in Northern India
*Corresponding author: Ujjala Ghoshal, Department of Microbiology, All India Institute of Medical Sciences, Kalyani, West Bengal, India. ujjalaghoshal@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: Ghoshal U, Singh R, Tejan N, Sahu C, Pandey A, Ghoshal UC. A study to assess the prevalence and risk factors for Clostridioides difficile infection in patients with inflammatory bowel disease in a tertiary care hospital in Northern India. J Lab Physicians. 2024;16:267-71. doi: 10.25259/JLP-2023-3-5-(1657)
Abstract
Objectives:
The prevalence of Clostridioides difficile infection (CDI) is on rise among patients with inflammatory bowel disease (IBD). This study sought to describe the prevalence and risk factors of CDI in patients with IBD as compared to non-IBD controls.
Materials and Methods:
This was a prospective study conducted at a Department of Microbiology in collaboration with a Department of Gastroenterology. The patients with IBD and controls without IBD presenting with diarrhea were included in the study. The screening test for C. difficile infection was done by glutamate dehydrogenase (GDH) assay and toxin detection by enzyme-linked immunoassay (ELISA). Anaerobic culture for C. difficile was done on a selective cycloserine cefoxitin fructose agar and polymerase chain reaction (PCR) was done for Toxin A (TcdA) and Toxin B (TcdB) gene detection. C. difficile infection was confirmed if GDH and toxin ELISA or PCR were positive.
Statistical Analysis:
Data were analyzed with the Statistical Package for the Social Sciences version 20.0.The numerical variables were presented by means and standard deviations. Comparison of continuous variables was done using Student’s t-test. Categorical variables were analyzed by Chi square test. P<0.05 was considered to be statistically significant.
Results:
A total of 160 cases and 112 age- and gender-matched control were included in IBD group and nonIBD group, respectively. Only one culture was positive, 12 and six were positive for GDH ELISA and TcdA and TcdB ELISA, respectively, and 7 were positive by PCR for toxin genes. The factors found significantly associated with CDI were proton-pump inhibitors use (P = 0.001), levofloxacin (P =0.001), and azathioprine (P =0.042). Using PCR as a reference method for C. difficile toxin detection, the sensitivity, and specificity of GDH ELISA and ELISA for toxins were 100%, 96.8% and 85.7%, and 100%, respectively.
Conclusions:
The prevalence of CDI among patients with IBD has been found to be low, that is (only 4.4%) in this study population.
Keywords
Clostridioides difficile
Inflammatory bowel disease
Prevalence
INTRODUCTION
Inflammatory bowel disease (IBD) is an entity that includes two distinct conditions, ulcerative colitis (UC) and Crohn’s disease (CD). The main characteristics of these two conditions are the chronic relapsing inflammation of the gut in individuals who are genetically predisposed, and exposed to defined environmental risk factors.[1,2] According to the European Crohn’s and Colitis Foundation, all patients with IBD who are on corticosteroids, immunomodulators, and biological agents should be considered immunocompromised and at increased risk for opportunistic infections.[3] In North America and Europe, over the past several decades, the prevalence of Clostridioides difficile infection (CDI) in IBD patients has increased rapidly.[4] The clinical presentation of CDI varies widely and ranges from an asymptomatic carriage to fulminant colitis with toxic megacolon. However, the global epidemiology remains undetermined due to insufficient data from underdeveloped countries. In a retrospective study conducted by Vaishnavi et al., from a tertiary care hospital in India, 19% of patients with IBD were positive for C. difficile toxin.[5] This study aims at enhancing awareness and improving the knowledge of CDI in IBD patients, which are important elements to optimize patient outcomes to implement preventive or early diagnostic and therapeutic strategies.
MATERIALS AND METHODS
This study was conducted in the Department of Microbiology in association with the Department of Gastroenterology from November 2017 to September 2019. Stool samples from 160 IBD cases, classified as UC and CD, were processed, diagnosed by clinical and endoscopic findings as per the World Gastroenterology Organization Global Guidelines on IBD.[6] The severity of UC was classified into mild, moderate, and severe using the Truelove and Witt’s criteria.[7,8] In this study, 112 patients without IBD but presenting with diarrhea were enrolled as controls. The demographic, clinical, and laboratory details of the patients were collected in a predesigned questionnaire. Anaerobic culture was put up on selective Cycloserine Cefoxitin Fructose Agar (CCFA) after alcohol shock and screening for glutamate dehydrogenase (GDH) was done by Meridian Bioscience Premier GDH kit while toxin detection was done by A/B by Meridian Bioscience Premier toxin A and B kit. For molecular detection, polymerase chain reaction (PCR) was done for TcdA and TcdB gene detection. For Toxin A (601bp) Forward Primer: YT28 - 5'CGATGATAAGGCAACTTCAGTGGTA 3' Reverse Primer: YT29 - 5' GAGTAAGATTCCTCCTGCTCCATTCAA 3'. For Toxin B (399 bp) Forward Primer: YT17 - 5' GGTGGAGCTGCTTCATTGGAGAG 3'Reverse Primer: YT18 - 5' GTGTAACCTACTTTCATAACACCA 3'.The reaction mixture contained 1x buffer (10 M Tris-HCL, 50 m MKCL, 1.5mM MgCl2), 200 pmol of each deoxynucleoside triphosphate (MBI, ferments), 20 pmol of Toxin A and B primers, and 1.25 U of Taq polymerase (Bangalore gene, India). The template was denatured for 5 min at 94°C, and DNA was amplified for 30 cycles consisting of 1 min at 94°C, 1 min at 52°C, and 7 min at 72°C. The DNA fragments of C. difficile amplified by PCR were identified by agarose gel electrophoresis.[9] C. difficile infection diagnosis was made as per the American College of Gastroenterology guidelines.[10]
Statistical methods
The data were analyzed with the Statistical Package for the Social Sciences version 20.0. The numerical variables were presented as by means and standard deviations. Comparison of continuous variables was done using Student’s t-test. Categorical variables were analyzed by Chi-square test. P < 0.05 was considered to be statistically significant.
RESULTS
A total of 160 IBD patients were included in this study, out of which 24 had CD and 136 had UC. 45% patients were females, and 55% were males. The mean age of IBD patients was 37.19 ± 16 years. In this study, 7 out of 160 IBD patients and none of the non-IBD group had CDI confirmed by PCR (P = 0.04). The prevalence of CDI in IBD patients in this study was 4.4%. Predominant clinical symptoms present in IBD patients were diarrhea (98.75%), followed by blood in stool in 94.4%, fever in 8.12%, abdominal pain in 11.9%, and vomiting in 2.5%. Out of these, fever was significantly associated with CDI as compared to patients without CDI (P = 0.001).
In this study, CDI in IBD patients was detected by culture on selective CCFA media, GDH enzyme-linked immunoassay (ELISA), Toxin ELISA, and PCR. Out of 160 patients, only 1 (0.6%) culture was positive, 12 were positive for GDH by ELISA, 6 were positive for TcdA and TcdB by ELISA, and 7 were positive by PCR for toxin gene [Figure 1 a and b]. Among the control group, culture and PCR were negative in all, ELISA was positive in 9 (8%) patients. The sensitivity, specificity, positive predictive value, and negative predictive value of various tests are shown in Table 1.
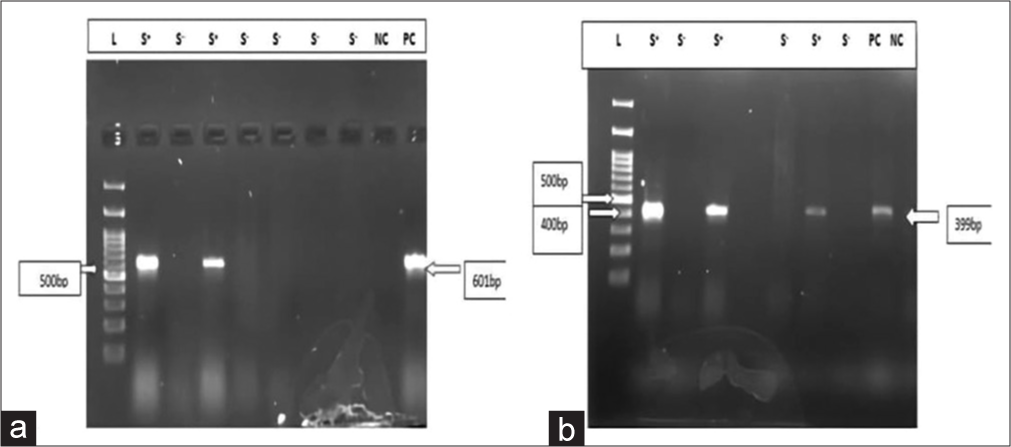
- (a) Gel doc picture showing toxin A gene; Lane 1- Ladder, Lane 2- sample, Lane 3- sample, Lane 4- sample, Lane 5- sample, Lane 6- sample, Lane 7- sample, Lane 8-sample, Lane 9- negative control, and Lane 10- positive control. (b) Gel doc picture showing toxin B gene; Lane1- Ladder, Lane 2- sample, Lane 3- sample, Lane 4- sample, Lane 5- sample, Lane 6- positive control, Lane 7- sample, Lane 8- positive control, and Lane 9- negative control.
| Testing methods | Sensitivity | Specificity | PPV | NPV | TAT (hours) |
|---|---|---|---|---|---|
| GDH | 100 | 96.8 | 58.3 | 100 | 2 |
| Toxin | 85.7 | 100 | 100 | 99.3 | 2 |
| Culture | 14.28 | 100 | 100 | 96.2% | 48 |
| PCR | Reference method for detection of C. difficile toxin | 48 | |||
NPV: Negative predictive value, PPV: Positive predictive value, C. difficile: Clostridioides difficile, GDH: Glutamate dehydrogenase, PCR: Polymerase chain reaction, TAT: Turn around time
The risk factors, including use of medications such as 5 amino salicylic acid (5-ASA), levofloxacin, cephalosporins, proton-pump inhibitors (PPIs), metronidazole, infliximab, and steroids were studied. Out of 7 toxin-positive cases by PCR, 5 were on azathioprine, 6 on mesalamine, 5 on levofloxacin, 2 on cephalosporin, 1 on infliximab, 5 on steroids, 5 on PPI, and none was on metronidazole. The factors found significantly associated with CDI were PPI use (P = 0.001), levofloxacin (P = 0.001), and azathioprine (P = 0.042), as shown in Table 2.
| Medication use | IBD with CDI | IBD without CDI | P-value |
|---|---|---|---|
| Levofloxacin | 5 | 7 | 0.001 |
| Cephalosporin | 2 | 12 | 0.116 |
| 5-ASA | 6 | 135 | 0.959 |
| Steroids | 5 | 93 | 0.721 |
| Infliximab | 1 | 3 | 0.168 |
| PPI | 5 | 18 | 0.001 |
| Azathioprine | 4 | 31 | 0.042 |
| Metronidazole | 0 | 8 | 0.535 |
IBD: Inflammatory bowel disease, CDI: Clostridioides difficileinfection, 5-ASA: 5 amino salicylic acid, PPI: Proton-pump inhibitors
Clinical outcomes of patients with CDI: Out of 7 CDI patients, only 1 was outpatient, and 6 were inpatients. The maximum duration of hospitalization among patients with CDI was 14 days,with a mean duration of 7.83 days. In this study, among the total GDH-positive cases, 25% were UC in remission, 25% had UC relapse, 33% had severe UC, and 17% were CD patients [Table 3]. All toxin-positive cases presented as IBD flare, and none was in remission. All CDI patients received oral vancomycin as treatment and these patients subsequently recovered.
| S. No. | GDH | Toxin | Culture | PCR | Clinical profile |
|---|---|---|---|---|---|
| 1. | Positive | Positive | Negative | Toxin B | Severe UC |
| 2. | Positive | Negative | Negative | Negative | Crohns |
| 3. | Positive | Negative | Negative | Negative | UC in remission |
| 4. | Positive | Negative | Negative | Negative | UC in remission |
| 5. | Positive | Negative | Negative | Negative | Crohns |
| 6. | Positive | Negative | Negative | Negative | UC in remission |
| 7. | Positive | Positive | Positive | Toxin B | Severe UC |
| 8. | Positive | Positive | Negative | Toxin A | Severe UC |
| 9. | Positive | Positive | Negative | Toxin B | UC relapse |
| 10. | Positive | Positive | Negative | Toxin A | UC relapse |
| 11. | Positive | Negative | Negative | Toxin B | Severe UC |
| 12. | Positive | Positive | Negative | Toxin A | UC relapse |
GDH: Glutamate dehydrogenase, PCR: Polymerase chain reaction, UC: Ulcerative colitis, ELISA: Enzyme-linked immunoassay
Comparison of IBD versus non-IBD group
The CDI prevalence was significantly increased in IBD group compared to non-IBD group, (P < 0.05). Symptoms including fever, abdominal pain, and blood in stool were found to be significantly more prevalent in IBD than non-IBD group (P < 0.001). A significant higher usage of antibiotics was observed in non-IBD group as compared to IBD group (P < 0.001). However, the use of levofloxacin and CDI was found to be significant only in IBD group (P < 0.001). Table 4 shows the comparison between IBD and non-IBD group.
| Variables | IBD (n=160) |
Non-IBD (n=112) |
P-value |
|---|---|---|---|
| Female: Male | 72:88 | 43:69 | 0.042 |
| Age year, (mean±SD) | 37.19±16 | 41±18.89 | 0.089 |
| C. difficile positive | 7 | 0 | 0.045 |
| Watery diarrhea | 158 | 102 | 0.523 |
| Abdominal pain | 62 | 19 | <0.001 |
| Fever | 78 | 14 | <0.001 |
| Blood in stool | 152 | 7 | <0.001 |
| Antibiotic usage | 34 | 37 | 0.008 |
C. difficile: Clostridioides difficile, IBD: Inflammatory bowel disease, SD: Standard deviation
DISCUSSION
To the best of our knowledge, this is the largest prospective study on C. difficile infection in patients with IBD in India. There are many studies on C. difficile infection in hospitalized patients, but very few studies have been conducted emphasizing CDI in IBD patients. We found the prevalence of CDI in IBD patients 4.4%, which is less as compared to other studies. There are very few studies from India on C. difficile infections in IBD patients. Kochhar et al., found that C. difficile toxin was present in 6 of 25 (24%) of patients with active colitis. Balamurugan et al. found that C. difficile was detected in 34 of 37 patients with UC.[11,12] These studies included a small number of patients. Another study by Vaishnavi et al., found a prevalence of 19% by toxin detection among patients with IBD, but it was a retrospective study, and only ELISA for toxins was done for diagnosis.[5] The reason for the low prevalence rates observed in our study might be that we have used PCR as a confirmatory method, which clearly ruled out false positive test results.[13]
The results of our study are in accordance with the study by Masclee et al., which reported toxigenic C. difficile in 3.6% IBD patients.[14] In another study by Gillespie et al., from the USA, the incidence of CDI among IBD patients was 6.7% among 654 IBD patients, which is closer to our study.[15] Another case–control study by Li et al. 2018 reported 7.41% of CDI cases among IBD patients.[16]
In our study, all the CDI cases had UC, but none had CD. Risk factors that were found to be significantly associated with the development of CDI in IBD patients in this study were use of PPI (P = 0.001), fluoroquinolone (P = 0.001), and azathioprine (P = 0.042). However, there was no significant relationship between the use of infliximab, 5-ASA, and steroids. Janarthanan et al., showed a significant relationship between CDI and PPI therapy, which is consistent with our study; while in contrast, Bossuyt et al., did not find any association between PPI use and CDI in IBD patients.[17,18] In our study, significant association between azathioprine use and CDI in IBD, which is in accordance with the study by Issa et al., which reported a greater than a two-fold increased risk of CDI with maintenance immunomodulator azathioprine.[19] Marwick et al., showed fluoroquinolones, which are widely used in IBD as a risk factor for CDI similar to findings in our study.[20] Schneeweiss et al. reported that the risk of CDI tripled with corticosteroid initiation among the IBD patients, while no such association was found with the initiation of immunomodulators or biologics (infliximab).[21] Similarly, there is no study from India that had compared the sensitivity and specificity of different testing methods for CDI. In this study, we found that GDH has high sensitivity so it can be used for exclusion of C. difficile infection or carriage. This is in accordance with a study by Swindells et al.,[22] which showed toxin detection by ELISA methods had lower sensitivity. Moreover, the most appropriate diagnostic results are obtained with PCR following combined antigen and toxin detection.[22]
The strength of this study is a prospective design in which consecutive patients were enrolled. Most of the previous studies have used retrospectively collected cases. A multistep algorithm is used for diagnostic approaches of C. difficile infection in symptomatic patients. However, one of the limitations of the study is the toxigenic culture,which is considered as gold standard for toxin detection, was not done as it is not suitable for routine diagnosis since it is a time-consuming and labor-intensive method. In our study, PCR is taken as a reference method for detection of toxin. In our study, diagnosis of C. difficile infection was made by laboratory diagnosis of toxigenic strain confirmed by PCR along with clinical symptoms. All patients with C. difficile infection were relieved of the symptoms by oral vancomycin. In addition, we performed PCR in all cases, which is considered as the highly sensitive method for confirmation of C. difficile infection, whereas the previous studies have diagnosed CDI using toxin ELISA,which has a low sensitivity. Therefore, in this study, the overall prevalence of CDI in patients with IBD was relatively more precise.
CONCLUSIONS
An IBD flare and CDI have similar presentations; therefore, an early diagnosis is important. In this study, GDH ELISA had high sensitivity and low specificity. GDH ELISA has low cost, thus making it appropriate for a screening test for CDI. More studies are required to determine whether the patients with negative GDH and toxin enzyme immunoassay results and positive for toxin PCR results should be treated or not. However, this prospective study provides significant data on the prevalence of CDI in IBD in Indian patients, associated risk factors, and sensitivity and specificity of different testing methods.
Acknowledgement
UG acknowledges ESCMID for its support.
Ethical approval
The Institutional Review Board and Ethics clearance was obtained (No. 2016-134-EMP-EXP).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
Dr. Chinmoy Sahu is on the Editorial Board of the Journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
The study was funded by an intra-mural grant from SGPGI, Lucknow.
References
- Inflammatory bowel disease: Established and evolving considerations on its etiopathogenesis and therapy. J Dig Dis. 2010;11:266-76.
- [CrossRef] [Google Scholar]
- Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8:443-68.
- [CrossRef] [Google Scholar]
- Clostridium difficile infection worsen outcome of hospitalized patients with inflammatory bowel disease. Sci Rep. 2016;6:29791.
- [CrossRef] [Google Scholar]
- Clinical and demographic profile of patients reporting for Clostridium difficile infection in a tertiary care hospital. Indian J Med Microbiol. 2015;33:326-7.
- [CrossRef] [Google Scholar]
- World gastroenterology organisation global guidelines inflammatory bowel disease: Update August 2015. J Clin Gastroenterol. 2016;50:803-18.
- [CrossRef] [Google Scholar]
- Guidelines for the management treatment of ulcerative colitis in Japan. IBD Res. 2010;4:189-239.
- [Google Scholar]
- Prevalence and association of PCR ribotypes of Clostridium difficile isolated from symptomatic patients from Warsaw with macrolidelincosamide-streptogramin B (MLSB) type resistance. J Med Microbiol. 2006;55:207-13.
- [CrossRef] [Google Scholar]
- ACG clinical guidelines: Prevention, diagnosis, and treatment of Clostridioides difficile infections. Am J Gastroenterol. 2021;116:1124-47.
- [CrossRef] [Google Scholar]
- Role of infectious agents in exacerbations of ulcerative colitis in India. J Clin Gastroenterol. 1993;16:26-30.
- [CrossRef] [Google Scholar]
- Estimation of faecal carriage of Clostridium difficile in patients with ulcerative colitis using real time polymerase chain reaction. Indian J Med Res. 2008;127:472-7.
- [Google Scholar]
- False-positive Clostridium difficile in negative-control reactions peak and then decrease with repetitive refrigeration of immunoassay. Int Sch Res Notices. 2014;2014:128120.
- [CrossRef] [Google Scholar]
- Is Clostridium difficile associated with relapse of inflammatory bowel disease? Results from a retrospective and prospective cohort study in the Netherlands. Inflamm Bowel Dis. 2013;19:2125-31.
- [CrossRef] [Google Scholar]
- Clostridium difficile in inflammatory bowel disease: A retrospective study. Gastroenterol Res Pract. 2017;2017:4803262.
- [CrossRef] [Google Scholar]
- Case-control study of inflammatory bowel disease patients with and without Clostridium difficile infection and poor outcomes in patients coinfected with C. difficile and Cytomegalovirus. Dig Dis Sci. 2018;63:3074-83.
- [CrossRef] [Google Scholar]
- Clostridium difficileassociated diarrhea and proton pump inhibitor therapy: A meta-analysis. Am J Gastroenterol. 2012;107:1001-10.
- [CrossRef] [Google Scholar]
- Increasing incidence of Clostridium difficileassociated diarrhea in inflammatory bowel disease. J Crohns Colitis. 2009;3:4-7.
- [CrossRef] [Google Scholar]
- Impact of Clostridium difficile on inflammatory bowel disease. Clin Gastroenterol Hepatol. 2007;5:345-51.
- [CrossRef] [Google Scholar]
- Community-associated Clostridium difficile infection among older people in Tayside, Scotland, is associated with antibiotic exposure and care home residence: Cohort study with nested case-control. J Antimicrob Chemother. 2013;68:2927-33.
- [CrossRef] [Google Scholar]
- Infliximab and other immunomodulating drugs in patients with inflammatory bowel disease and the risk of serious bacterial infections. Aliment Pharmacol Ther. 2009;30:253-64.
- [CrossRef] [Google Scholar]
- Evaluation of diagnostic tests for Clostridium difficile infection. J Clin Microbiol. 2009;48:606-8.
- [CrossRef] [Google Scholar]






