Translate this page into:
Acantholytic squamous cell carcinoma of larynx: An unusual cause of asphyxial death diagnosed on autopsy
*Corresponding author: Kavita Gaur, Department of Pathology, Lady Hardinge Medical College, New Delhi, India. kavgaur@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Osama MA, Gaur K, Agarwal K, Sekar L, Singh S, Singh S. Acantholytic squamous cell carcinoma of larynx: An unusual cause of asphyxial death diagnosed on autopsy. J Lab Physicians. doi: 10.25259/JLP-2023-9-9-(1948)
Abstract
Acantholytic squamous cell carcinoma (ASCC) is an uncommon histopathological variant of squamous cell carcinoma (SCC). This tumor commonly occurs in the sun exposed areas of skin at the head and neck location. The possible occurrence at mucosal sites has seldom been reported. Although, this malignant tumor has an excellent prognosis in the skin, in sharp contrast, mucosal involvement carries a worse prognosis. Here, we explore an intriguing case of an asphyxial death of a 35-year-old man due to a polypoidal mass occluding the airway in the glottic region. Subsequently, based on the histopathological and immunohistochemical findings, the cause of death was diagnosed as ASCC based on histopathological and immunohistochemical findings. Furthermore, this report also highlights the rarity of this variant of SCC at an uncommon location, thus making it the third case ever reported in literature. The other interesting fact about this case is that most of the published reports in the literature have described and diagnosed this rare variant on antemortem examination; contrarily, a postmortem diagnosis of ASCC is exceptional, making it the first report ever in history.
Keywords
Acantholytic
Autopsy
Epithelial-cadherin
Squamous cell carcinoma
Larynx
Mucosa
Postmortem
Vimentin
INTRODUCTION
Laryngeal malignancies constitute 13% of all the head and neck region cancers.[1] Principally, laryngeal malignancies are majorly of squamous cell lineage. Squamous cell carcinoma (SCC) is classified into two groups according to the pathological features: conventional (classic) type (95%) and variants of SCC (5%).[2] SCC has quite a few variants, namely, conventional SCC (48–65%), basaloid carcinoma (4–10%), warty carcinoma (7–10%), papillary carcinoma (5–15%), verrucous carcinoma (3–8%), and mixed carcinomas.[3] Each subtype has a distinct histomorphological feature. Acantholytic SCC (ASCC) represents a rare subset (2–4%) of SCC characterized by acantholysis of the tumor cells, creating pseudo lumina, and false appearance of glandular differentiation.[4] Awareness of the diagnostic nuances of ASCC is indispensable for an accurate diagnosis as they exhibit deceptive morphological and immunohistochemical profile.
CASE REPORT
A 35-year-old young man was found unconscious on the railway station platform, following which he was taken to the hospital, where he was declared dead. On introspection with his family members, it was discerned that the individual had a recurring history of vocal cord polyps over the past year. He had experienced challenges encompassing both dysphagia and respiratory distress at night. He had undergone several microlaryngeal procedures, with the most recent polyp removal performed through carbon dioxide laser ablation, which unexpectedly was histopathologically diagnosed as a benign vocal cord nodule. A post mortem examination was sought to obtain insights and determine the precise cause of the abrupt demise. On autopsy, the pharynx, base of tongue, larynx, and both vocal cords were markedly congested. The lungs were turgescent and edematous. A large polypoidal mass occluding the airways was identified in the laryngeal lumen; thus, total laryngectomy along with partial glossectomy specimen was sent for histopathological examination. The entire specimen in-toto measured 15.5 × 8.5 × 5 cm. On opening, a large polypoidal, exophytic mass measuring 3 × 1 × 1.2 cm was seen in the glottic space [Figure 1]. The mucosa over the polyp and the surrounding mucosa appeared ulcerated. The mass was restricted to the midline; however, no extension into the right and left sides of the larynx was seen grossly. Sections studied from the polyp in the glottic region showed nests of malignant squamoid cells, displaying an infiltrative growth pattern. Individual cells showed increased nuclear-cytoplasmic ratio, hyperchromasia, irregular nuclear membrane, and dense eosinophilic cytoplasm. Within the tumor nests and islands, intercellular bridges, mitotic figures, and intracellular keratin along with keratin pearl formation were identified [Figure 2a]. In some foci, malignant squamous cells appeared dyscohesive and were seen scattered throughout the tumor substance [Figure 2b-f]. Periodic acid-Schiff stain negated the presence of any mucinous component. Right, left, and posteroinferior mucosal margins of the specimen showed only mild dysplasia; however, no frank tumor was seen in any of the margins or the adjacent structures. The tumor remained confined to the midline glottic space. On the basis of morphological features, a diagnosis of acantholytic variant of SCC was favored. A panel of immunohistochemical stains were put, to confirm this variant of SCC. To confirm the lineage of these malignant cells, cytokeratin 5/6 was applied, which showed diffuse cytoplasmic staining and confirmed them to be of squamous lineage [Figure 3a]. Epithelial-cadherin (E-cadherin) showed loss of expression in the malignant squamous cells, and vimentin positivity in the cells confirmed the epithelial mesenchymal transition (EMT) in the cells [Figure 3b and c]. Thus, a final diagnosis of SCC, acantholytic variant was proffered on postmortem examination.
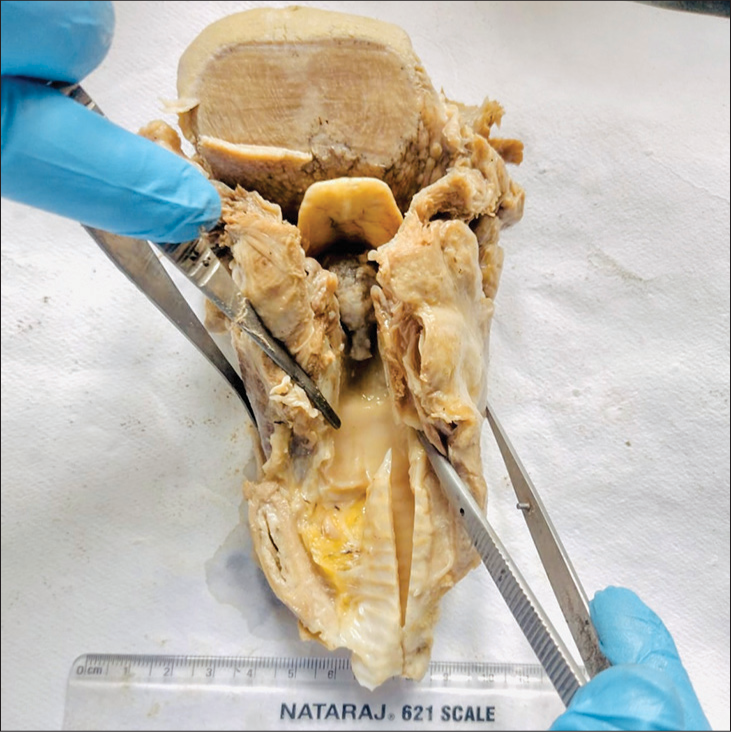
- Gross image showing an a polypoidal pedunculated mass present in the midline of the glottic region (autopsy specimen).
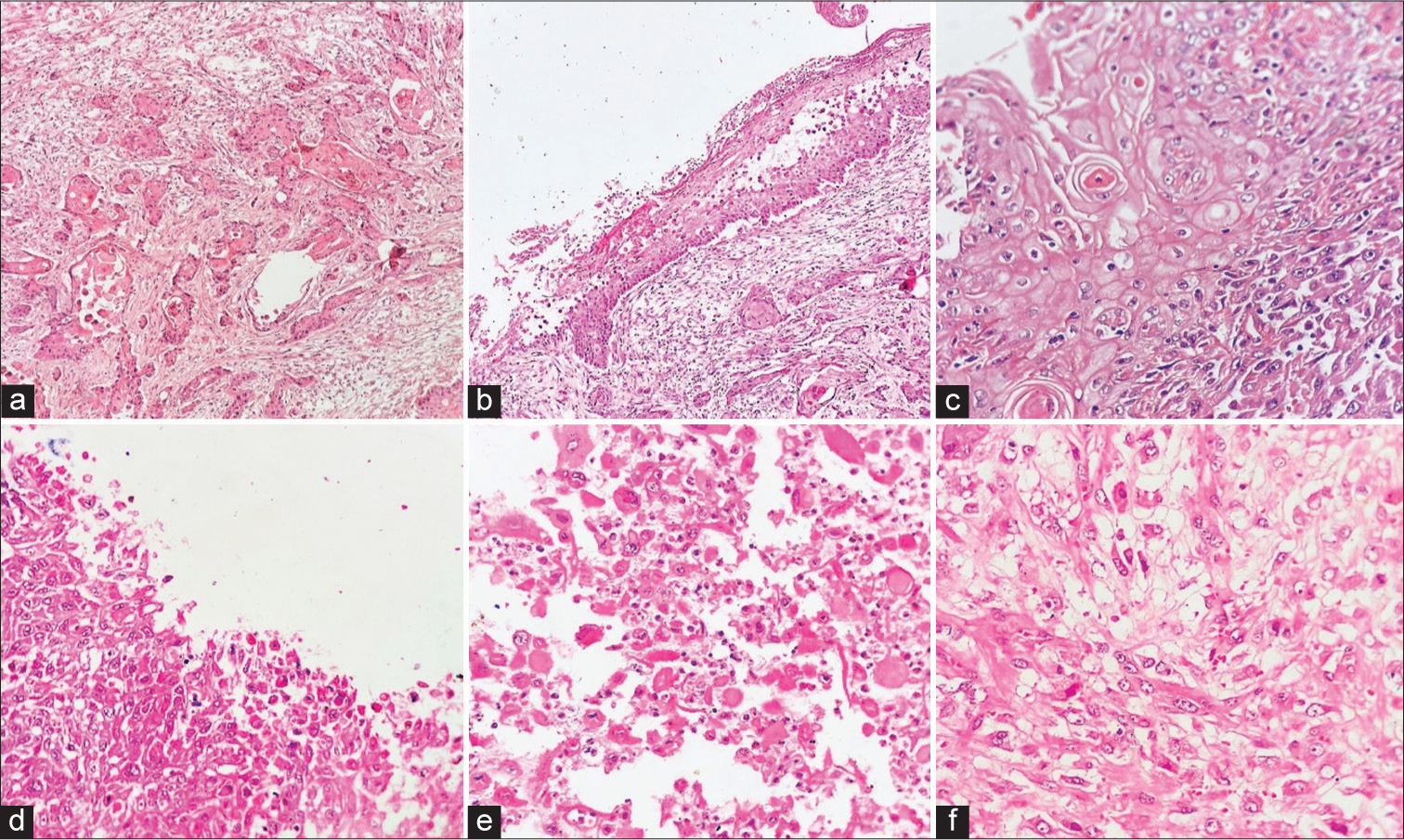
- (a) Malignant tumor showing nests of squamoid cells, displaying an infiltrative growth pattern. Tumor nests and islands showing intercellular bridges and keratin pearl formation (HE ×40), (b and c) individual cells showing hyperchromasia, irregular nuclear membrane, and dense eosinophilic cytoplasm (HE ×40, ×100), and (d-f) malignant squamous cells appeared round to oval (acantholytic cells) and dyscohesive in nature, scattered throughout the tumor substance (HE ×100, ×200, ×400). HE: hematoxylin and eosin.
![IHC- (a) CK5/6: Positive (×40) (Inset- high-power image [×200]), (b) Epithelial-cadherin: Negative (×40), (c) IHC: Vimentin positive (×100) (Inset- high-power image [×200]). IHC: Immunohistochemistry.](/content/164/2024/0/1/img/JLP-2023-9-9-g003.png)
- IHC- (a) CK5/6: Positive (×40) (Inset- high-power image [×200]), (b) Epithelial-cadherin: Negative (×40), (c) IHC: Vimentin positive (×100) (Inset- high-power image [×200]). IHC: Immunohistochemistry.
DISCUSSION
ASCC, a subtype of SCC, has been known by various denominations such as, pseudoglandular squamous carcinoma, angiosarcoma-like SCC, and adenoid squamous carcinoma. ASCC was first coined by Lever in 1947 and described them by the proliferation of keratinocytes, which are variably-sized.[5] These cells are dyscohesive in nature and, thus, form gland-like structures with these acantholytic cells. Although, head and neck region are the usual site, the possible occurrence at the mucosal sites of oral cavity, nasopharynx, tongue, and especially larynx has hardly been described in the literature.[6,7] This variant of SCC shows non-solid components. This is typically characterized by free floating single or groups of acantholytic or dyskeratotic malignant epithelial cells. Thereby, forming pseudoglandular or pseudovascular structures. The tumor cells of ASCC, contrary to conventional SCC, shows a significant amount of pleomorphism. Thus, appearing extremely bizarre as typically seen in poorly differentiated malignant tumors. Features such as irregular nuclear membrane, hyperchromasia, and prominent nucleoli are quite often seen in ASCC. Brisk mitosis, along with atypical forms is frequently seen. The cells show variable amount or dense eosinophilic cytoplasm. There is an absence of epithelial mucin production (so as to differentiate from adenosquamous variant of SCC).
These cells of ASCC lose the quintessential attributes of conventional SCC, simulating other epithelial or mesenchymal malignancies. The cells lose intercellular junctions and cellular polarity, acquire mesenchymal properties, and gain increased motility, leading to EMT. E-cadherin is a cell adhesion molecule,and a tumor suppressor protein plays a significant role in EMT. It acts as a morphogenetic controller by altering the tissue maturation and tumor differentiation. In normal conditions, E-cadherin is present in nearly all developed epithelial cells of human tissue and many solid malignant epithelial tumors; however, its expression is typically decreased in a few tumors like lobular carcinoma of the breast. The tumor cells of ASCC show significant down regulation of E-cadherin along with an up regulation of Vimentin (mesenchymal marker), Claudin-1 (tight junction protein), bringing about EMT, and invasion of tumor cells.[8] Griffin et al. noted that E-cadherin expression was significantly reduced in ASCC, compared with SCC.[9]
Clinically, the head and certain facial locations (lips) are favored by ASCC. Conversely, distal sites on both the upper and lower limbs are favored by conventional SCC. Cunha et al.,[10] in their study, compared the tumor behavior of conventional SCC with ASCC,and found a higher tumor grade, deeper infiltration, and vascular invasion in ASCC than in typical SCCs. Furthermore, they observed a higher incidence of regional, lymph nodal metastasis, and mortality due to the aggressive behavior of ASCC.[10] Similarly, Bayer-Garner et al. found that the reduced expression of E-cadherin was associated with a poor prognosis in ASCC cases.[11] Factors such as tracheal mucosal infiltration and poor-differentiation of tumor are considered as unfavorable prognostic and predictive factors. This tumor variant being rare in itself, has hardly been described at larynx, thus leading to a limitation on information of pathological, immunohistochemical findings, and prognosis [Table 1].
| Author | Age/sex | Location | Antemortem/Postmortem diagnosis | Treatment | Follow-up |
|---|---|---|---|---|---|
| González-Vela et al. (2006)[6] | 75 years/M | Right vocal cord | Antemortem | Total laryngectomy, radical neck dissection and chemotherapy | Died after 9 months |
| Ripanti et al. (2019)[7] | 51 years/M | Subglottis | Antemortem | Total laryngectomy with radical neck dissection | 2 months, Recurrence |
| Present case (2023) | 35 years/M | Glottis (midline) | Postmortem | Post mortem (total laryngectomy along with partial glossectomy) | Died |
CONCLUSIONS
In conclusion, the report emphasizes that ASCC is an uncommon subtype of SCC, rarely observed in mucosal locations, particularly within the larynx. Given the tumor’s inherently aggressive nature, resulting in a fatal outcome, occurrences of this nature are exceedingly rare, underscoring the significance of this documentation.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate consent from the patient’s relatives for featuring the deceased patient’s images and clinical information and non-publication of the name and initials in all formats of the Journal.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries [published correction appears in CA Cancer J Clin 2020;70:313] CA Cancer J Clin. 2018;68:394-424.
- [CrossRef] [PubMed] [Google Scholar]
- Laryngeal dysplasia, squamous cell carcinoma, and variants. Surg Pathol Clin. 2017;10:15-33.
- [CrossRef] [PubMed] [Google Scholar]
- Advances in the pathology of penile carcinomas. Hum Pathol. 2012;43:771-89.
- [CrossRef] [PubMed] [Google Scholar]
- Adenoid squamous cell carcinoma of oral cavity: A case report. J Dent (Shiraz). 2018;19:68-73.
- [Google Scholar]
- Adenocanthoma of sweat glands; carcinoma of sweat glands with glandular and epidermal elements: Report of four cases. Arch Derm Syphilol. 1947;56:157-71.
- [CrossRef] [PubMed] [Google Scholar]
- Adenoid squamous cell carcinoma of the larynx: An uncommon histological variant of squamous cell carcinoma. APMIS. 2006;114:470-3.
- [CrossRef] [PubMed] [Google Scholar]
- A rare case of acantholytic squamous cell carcinoma of the larynx. Front Physiol. 2019;10 doi: 10.3389/conf.fphys.2019.27.00011
- [CrossRef] [Google Scholar]
- Keratin down-regulation in vimentin-positive cancer cells is reversible by vimentin RNA interference, which inhibits growth and motility. Mol Cancer Ther. 2008;7:2894-903.
- [CrossRef] [PubMed] [Google Scholar]
- Decreased expression of intercellular adhesion molecules in acantholytic squamous cell carcinoma compared with invasive well-differentiated squamous cell carcinoma of the skin. Am J Clin Pathol. 2013;139:442-7.
- [CrossRef] [PubMed] [Google Scholar]
- Pseudoglandular (adenoid, acantholytic) penile squamous cell carcinoma: A clinicopathologic and outcome study of 7 patients. Am J Surg Pathol. 2009;33:551-5.
- [CrossRef] [PubMed] [Google Scholar]
- Syndecan-1 expression is diminished in acantholytic cutaneous squamous cell carcinoma. J Cutan Pathol. 1999;26:386-90.
- [CrossRef] [PubMed] [Google Scholar]






