Translate this page into:
Bacterial and fungal flora of conjunctiva of patients presenting with cataract and their seasonal variation in Northern India
*Corresponding author: Vinita Gupta, Department of Ophthalmology, All India Institute of Medical Sciences, Rishikesh, Uttarakhand, India. vinita2000gupta@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Gupta V, Naharwal A, Mood M, Kumar S, Shrestha S, Chand S. Bacterial and fungal flora of conjunctiva of patients presenting with cataract and their seasonal variation in Northern India. J Lab Physicians. doi: 10.25259/JLP-2023-1-11-(1541)
Abstract
Objectives:
The objective of this study was to study the microbial flora (bacterial and fungal) of the conjunctival sac of patients presenting with cataracts at a tertiary care hospital in North India.
Materials and Methods:
This observational cross-sectional study included 320 eyes from 238 patients presenting with cataracts. Three conjunctival swabs were collected from each eye and analyzed for the presence of aerobes, anaerobes, and fungal growth.
Statistical Analysis:
The data were analyzed using R statistical environment 4.0 software, along with the R Commander plugin “EZR.” Kruskal–Wallis rank sum test. In addition, the Fisher exact test was conducted, considering P < 0.05 as statistically significant.
Results:
A total of 75.63% (242) of conjunctival sacs were culture-positive, with a predominance of bacterial growth. On the other hand, 24.37% (78) of conjunctival sacs were sterile. Common commensals isolated included Staphylococcus epidermidis (34.06%), Staphylococcus aureus (10.0%), Propionibacterium spp. (7.5%), and Corynebacterium spp. (5.31%). There were 23 (10%) Gram-negative cultures, whereas three fungi were isolated, all of which were Candida spp. The highest total growths were observed in age group 2 (51–65 years); however, no statistically significant correlation existed between age groups and growths. Season 1, characterized by temperatures between 10° and 15°, exhibited maximum growths. The most growths were coagulase-negative staphylococci (CONS).
Conclusions:
Our study of North Indian eyes revealed that 75.63% of healthy conjunctival sacs showed positive cultures, mainly CONS, with no seasonal trend. Given CONS’s prevalence in post-cataract endophthalmitis, routine pre-operative conjunctival swabs could guide prophylaxis and lower rates of post-operative endophthalmitis.
Keywords
Coagulase- negative staphylococci
Conjunctival sac
Gram-positive and Gram-negative
Microbial growths
Post-cataract endophthalmitis
INTRODUCTION
Cataract surgery has become one of the most prevalent ophthalmic procedures due to changes in population structure and increased life expectancy. Post-operative bacterial endophthalmitis is often a devastating complication of cataract surgery, with an incidence varying between 0.029% and 0.29%.[1-3] The source of infection is usually the patient’s flora or the environment at the time of surgery. Microbes present on the lid margins may gain access to the anterior chamber by adhering to intraocular lenses and instruments, as well as through the entry of fluid from the operative field. Speaker et al. demonstrated the correlation between external flora and intraocular infections using genotypic analysis to identify the etiologic agent of acute post-operative endophthalmitis (PE).[4] They found that 82% of micro-organisms identified in the vitreous were genetically identical to the isolates recovered from the conjunctiva, eyelid, and nasal mucosa of the patients.
Hence, it is important to identify risk factors as well as the organisms responsible for PE. In most cases, however, the source cannot be definitively identified. Identifying the various commensals and pathogenic micro-organisms inhabiting the conjunctival sac of a patient undergoing cataract surgery may, therefore, help in identifying the source of infection in patients who develop PE. It may also enable the selection of a specific perioperative antibiotic for PE prophylaxis.
Wide variations in the isolation rates of normal conjunctival flora have been reported from different parts of the world, yet no specific cause has been attributed. Climatic and geographical variations, as well as the demographic profile of the population, may be important determinants for this variation. Therefore, we conducted a study to isolate and characterize the external flora of the conjunctival sac in patients presenting with cataracts. We also analyzed the effects of seasons and age on the growth pattern of the flora inhabiting the sac.
MATERIALS AND METHODS
The study was an observational cross-sectional and hospital-based study designed to adhere to the tenets of the Declaration of Helsinki. Approval from the Institutional Ethics Committee was obtained. A total of 238 consecutive patients aged 18 years and above, with cataracts in one or both eyes, presenting to the Ophthalmology Department of All India Institute of Medical Sciences, Rishikesh, India, from December 2016 to November 2017, were enrolled. Patients with active ocular infections such as infectious conjunctivitis and/or keratitis, those with a history of contact lens wear, individuals on topical anti-glaucoma medications, or those receiving topical or systemic antibiotics were excluded from the study. All the patients were followed up for surgical intervention.
The study period of 12 months, spanning from December 2016 to November 2017, was divided into four seasons: Season-1 (Winter, December–March, temperatures 10–15°C), Season-2 (Summer, April–July, temperatures 32–40°C), Season-3 (Monsoon, August–September, temperatures 20–30°C), and Season-4 (Autumn, October–November, temperatures 25–35°C), in accordance with the classification by the Meteorological Department of India.
Sterile-moistened cotton swabs were used to collect three conjunctival swabs from each eye with cataracts. Care was taken to avoid contact with the eyelids during sampling. The samples were promptly transported to the laboratory and inoculated without delay. The initial swab underwent both Gram staining and Zeihl-Neelsen staining, followed by inoculation onto chocolate, blood, and chromogenic agar (CPS3). Colonies that grew were further identified using kits, reagents, and differentiation discs following standard microbiological protocols.
For the second swab, inoculation was performed in Robertson-cooked meat media or thioglycolate media for anaerobic organisms. The third swab, after potassium hydroxide and lactophenol cotton blue mount examination, was inoculated on Sabraud’s dextrose agar for fungi.
The samples were categorized into three groups: Group-1 (eyes of patients aged 18–50 years), Group-2 (eyes of patients aged 51–65 years), and Group-3 (eyes of patients aged above 65 years).
The STROBE diagram [Figure 1] illustrates the total number of eyes included in the study, as well as the number of eyes that underwent cataract surgery at our hospital. They were among those with both sterile sacs and those with conjunctival sacs showing growth in initial swabs. Among the 320 eyes, those with swabs indicating no growth underwent cataract surgery, whereas eyes with positive growth were treated with specific antibiotics and scheduled for surgery. Operative cases were subsequently monitored for three months.

- STROBE flow diagram describing number of eyes included, operated, not operated, and those developing post-operative endophthalmitis. PE: Post-operative endophthalmitis; n: Number of patients.
The analysis of data, including the correlation between seasons and age with the growth pattern of the flora, was conducted using the R statistical environment version 4.0 software, along with the R Commander plugin “EZR.” The analysis involved the use of the Kruskal–Wallis rank sum test and the Fisher exact test. P < 0.05 was considered statistically significant.
RESULTS
Samples were collected from 320 conjunctival sacs of 138 males and 100 females (238 patients). Among these, 78 conjunctival sacs (24.37%) were found to be sterile, whereas 242 sacs (75.63%) displayed growths on cultures. The total number of Gram-positive bacteria, Gram-negative bacteria, fungi, and eyes with no growth is illustrated in Figure 2. The most frequent commensals isolated from the conjunctival sac were Staphylococcus epidermidis/Coagulase-negative Staphylococcus (CONS) (34.06%), Staphylococcus aureus (10.0%), Propionibacterium spp. (7.5%), and Corynebacterium spp. (5.31%). Gram-negative growths were observed in 23 eyes (10%), including Haemophilus influenzae, Klebsiella spp., Escherichia coli, Pseudomonas aeruginosa, Bacteroides spp., Moraxella spp., and Clostridium spp. Only three cases of fungi were isolated, all of which were Candida spp. [Table 1].
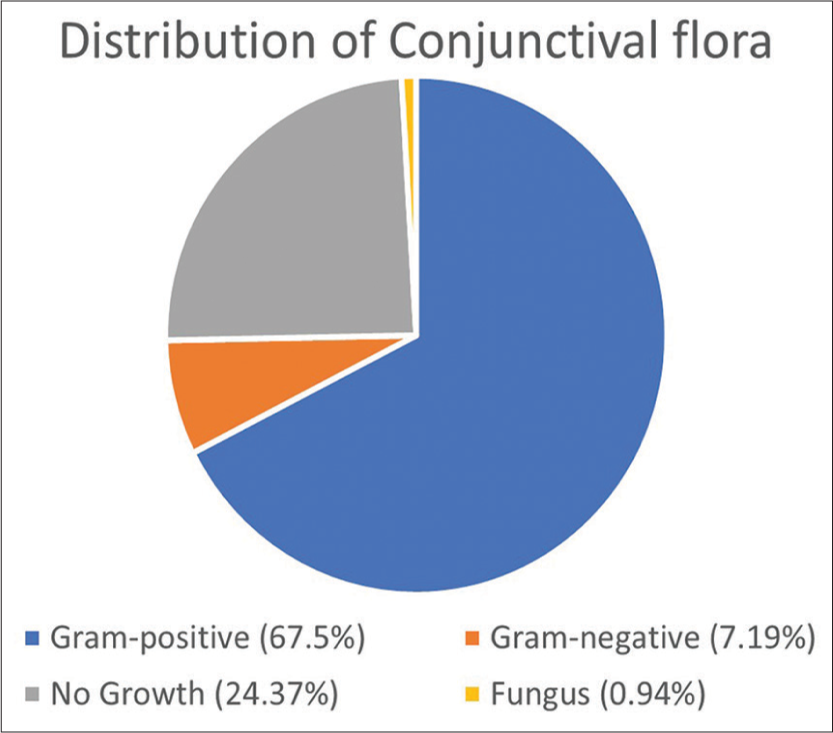
- Total number of growths - Gram-positive bacteria, Gram-negative bacteria, fungi, and eyes with no growth.
| Microorganism | Number of growths | Percentage of growths |
|---|---|---|
| Staphylococcus epidermidis | 109 | 34.06 |
| Staphylococcus aureus | 32 | 10.0 |
| Micrococcus | 9 | 2.81 |
| Streptococcus pyogenes | 4 | 1.25 |
| Streptococcus pneumoniae | 5 | 1.56 |
| Viridans streptococci | 2 | 0.62 |
| Moraxella catarrhalis | 8 | 2.5 |
| Corynebacterium spp. | 17 | 5.31 |
| Hemophilus influezae | 2 | 0.62 |
| Klebsiella spp. | 1 | 0.31 |
| Escherichia coli | 2 | 0.62 |
| Pseudomonas aeruginosa | 4 | 1.25 |
| Moraxella spp. | 5 | 1.56 |
| Propionibacterium spp. | 24 | 7.50 |
| Peptostreptococcus | 2 | 0.62 |
| Bacteroides spp. | 3 | 0.94 |
| Lactobacillus | 4 | 1.25 |
| Clostridium spp. | 5 | 1.56 |
| Fungi | 3 | 0.94 |
Values in bold represent the common commensals isolated from the conjunctival sac in their decreasing order of frequency expressed in numbers and percentages.
Eyes in Group-2 (51–65 years) exhibited the highest count of microbial growths, with 112 eyes affected. Of the 105 eyes showed Gram-positive organisms, and 7 displayed Gram-negative organisms. A total of seven eyes in both Groups 1 and 2 exhibited Gram-negative growths, while Group 3 had nine eyes with similar growths. The distribution of microbial growths across various age groups is illustrated in Figure 3. The ratio of Gram-positive to Gram-negative growths [Table 2] was not found to have a statistically significant difference across different age groups (P = 0.16).

- Distribution of the microbial growths in various age groups. S. epidermidis: Staphylococcus epidermidis.
| Age groups (years) | Gram-positives (%) | Gram-negatives (%) | Total |
|---|---|---|---|
| Group-1 (<50) | 61 (89.70) | 7 (10.29) | 68 |
| Group-2 (51–65) | 105 (93.75) | 7 (0.62) | 112 |
| Group-3 (>65) | 50 (84.7) | 9(15.25) | 59 |
The correlation of all growths with the seasons is depicted in Figure 4, and no discernible seasonal pattern was identified (P = 0.145). However, Gram-positive organisms exceeded Gram-negative organisms across all four seasons. A notable variation in the proportion of Gram-positive and Gram-negative growths was observed between different seasons (P = 0.04). The prevalence of Gram-positive growths was at its peak during Season-1, with temperatures ranging between 10°C and 15°C, and at its lowest during Season-2, with temperatures ranging between 32°C and 40°C. The prevalence of coagulase-negative Staphylococcus was highest during Season-1 (45 eyes) and the lowest during Season-2 (13 eyes). The maximum number of Gram-negative growths was recorded during Season-4 (11 eyes), with none during Season-2.

- Correlation of all growths with seasons.
DISCUSSION
The conjunctival sac becomes colonized by bacteria at birth, and it remains so throughout life with changes in the flora due to various factors.[5] Bacteria colonizing conjunctival sacs produce various bacteriocins and inhibitory products such as lactic and acetic acids, thus giving them the necessary competitive advantage to survive and prevent the establishment of pathogenic micro-organisms.[6] The microbial flora of the conjunctival sac mainly consists of S. epidermidis, S. aureus, Corynebacterium species, and Propionibacterium acnes.[7] With increasing age, Gram-negative organisms also become part of the flora.[5] The prolonged use of topical antibiotics results in changes in the microbial flora, leading to the colonization of fungal and antibiotic-resistant bacteria.[7,8]
PE is a devastating complication of intraocular surgery. Numerous risk factors for PE are described, linked to patients’ demographics, such as old age, rural residence, and immunosuppressive conditions like diabetes mellitus. Conditions such as blepharitis, ectropion, and those involving an increased number of ocular flora are associated with an increased risk of developing PE. Intraoperative risk factors reported include intracapsular cataract surgery, vitreous loss, and anterior vitrectomy. Moreover, it has been observed that the virulence and infectious dose of inoculated pathogens, along with their antibiotic sensitivity, play a significant prognostic role.[9]
The most common organisms in culture-positive post-cataract endophthalmitis remain Gram-positive bacteria, 70% of which are CONS. Other common bacteria include S. aureus (10%), Streptococcus species (9%), and Enterococcus species (2.2%). Gram-negative species account for about 5–6% of cultures and include Pseudomonas, Proteus, and Haemophilus influenzae.[10] Delayed post-cataract endophthalmitis is known to be most commonly caused by P. acnes.[11-14]
Although there are various studies pertaining to the normal microflora of conjunctival sacs all over the world; the data of one region cannot be extrapolated directly for every patient as several factors such as climatic and geographical variation affect the type and susceptibility pattern of bacteria. Arantes et al.[7] in their study of 50 Brazilian patients undergoing cataract surgery, found positive cultures in 86% of the eyes. Among these, 88.9% were Gram-positive, and 11.1% were Gram-negative. A study from Central Africa[15] demonstrated bacterial growth in 61.6% of conjunctival specimens, all of which were identified as Gram-positive. In contrast, a study from Nigeria (Western Africa)[16] reported a bacterial isolation rate of only 36.5%, with 73.7% being Gram-positive and 26.3% Gram-negative. Suto et al.[17] also found a bacterial isolation rate of 39.2% in 579 eyes of patients undergoing cataract surgery, with 94.01% being Gram-positive.
This wide variation has also been observed across the Indian subcontinent. In our study, we found that 24.37% (78 eyes) of conjunctival sacs were sterile, a result comparable to the findings of Sharma et al.[18] who reported 36% sterile sacs. However, Kudva et al.[19] and Karthika et al.[20] from South India reported higher percentages, with 50% and 48% sterile sacs, respectively. On the other hand, Saxena and Goswami[21] from North India did not find any sterile sacs in their series. In our study, we identified Gram-positive bacterial cultures in 90.3% of cases. These findings are consistent with results reported in the majority of studies.[18-21]
The most common commensals isolated in our study were S. epidermidis (CONS) at 34.06%, followed by S. aureus at 10.0%, Propionibacterium spp. at 7.5%, and Corynebacterium spp. at 5.31%. These findings are consistent with literature indicating that these bacteria are frequently implicated in PE, with CONS being responsible for the majority of cases.[11] Similar outcomes have been documented by Sharma et al.,[18] and other authors[19,22] who reported CONS-positive cultures in 43%, 80.5%, and 83.1% of cases, respectively. Furthermore, our study revealed positive cultures for P. acnes in 7.5% of eyes (24 cases). Binder et al.[23] also reported a similar finding of 23% positive swabs for P. acnes, which could, however, be reduced by 92% through the application of polyvidone iodine disinfection before cataract surgery.
Demographic characterization of the patients in our study revealed maximum growth of CONS and Gram-negative bacteria in Group-2 (51–65 years). Furthermore, in all the age groups, Gram-positive growths were more than Gram-negative. However, Liu et al.[5] found significant positive culture growth in the elderly as compared to the younger age group. Khorazo and Thompson[24] also observed that conjunctival sacs in the elderly are less often sterile than in the young. Sharma et al.[18] found this trend for S. aureus with a significant increase from 8.9% in the young to 16.7% in middle-aged and further to 23.1% in the elderly. However, the incidence of CONS in their study declined with increasing age. We could not find any such correlation in our study which may have been due to unequal distribution of the total number of study eyes in each group.
When analyzing the impact of seasons, our study revealed that Gram-positive organisms outnumbered Gram-negative organisms across all four seasons. Interestingly, no Gram-negative growths were observed during Season-3. Fungal growths were only identified during Season-3 and among patients aged 50 years and older. However, no statistically significant differences were observed in the growth patterns across different seasons, a result consistent with the findings of Khorazo and Thompson.[24]
The highest growth rates were recorded during Season-1, when temperatures ranged from 10°C to 15°C. Khalaila et al.[25] from Israel also reported that the highest number of patients developed conjunctivitis when the temperatures ranged from 13°C to 23°C, as demonstrated in their extensive study involving 601 patients with conjunctivitis. Rubio,[26] in their study exploring the climatic impact on the prevalence of conjunctival bacteria in patients undergoing cataract surgery, discovered that Gram-positive bacteria exhibited an increase at temperatures ranging from 15°C to 19°C (i.e., during the month of May). Moreover, they noted that the incidence of re-hospitalization for endophthalmitis following cataract extraction in May and June combined was 3.37 times higher compared to other months. This suggests that seasonal prevalence patterns in ocular surface flora might contribute as a predisposing factor for PE occurrence in certain seasons and months.
Studying micro-organisms inhabiting the conjunctival sac of cataract surgery patients may help in identifying the source of infection in patients who develop PE. This may enable selecting specific perioperative prophylactic antibiotics, especially for high-risk cases such as the elderly, diabetics, and the immunocompromised. Our study highlights a seasonal pattern in conjunctival flora growth, potentially linked to higher PE risk during certain months. These findings boost infection understanding and improve prophylactic strategies, benefiting patient care, and surgical procedures.
However, our study has several limitations. We inferred the relative health of the ocular surface in patients with cataracts through a review of symptoms, medical history, and external examinations. It is possible that some subjects had subclinical or asymptomatic disease (such as mild blepharitis), and as a result, their ocular surface might not have been truly healthy. Subsequent studies could investigate whether relatively mild ocular surface disease influences the resident microbiome. In our study, we solely utilized traditional culture methods. These methods yield cultivable microbes in <80% of conjunctival swabs.[27] Moreover, they have limitations in identifying coinfections involving fastidious, facultative, or obligate bacteria, and even non-cultivable ones.[28] Alternatively, molecular techniques such as polymerase chain reaction combined with cloning and DNA sequencing allow for the rapid identification of bacteria. Utilizing 16S ribosomal RNA gene libraries and sequencing, it becomes possible to detect even the difficult-to-culture bacterial community up to the species level.[29,30] These advanced methods possess the capability to analyze polybacterial infections from ocular samples with heightened sensitivity and provide reliable phylogenetic identification. Employing these techniques in cross-sectional and longitudinal studies opens up the possibility of determining the presence of non-cultivable microbes that can colonize the ocular surface. Moreover, our study did not establish a correlation between the outcomes of cataract surgeries in all eyes with positive cultures. This prevents us from accurately determining the exact incidence of PE caused by these microbes. Further studies are imperative to explore and elucidate this potential link.
CONCLUSIONS
While our study offers valuable insights, these limitations underscore the need for more comprehensive investigations in the field of ocular microbiomes and their implications for surgical complications.
Ethical approval
The research/study complied with the Helsinki Declaration of 1964.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Microbiological analysis of conjunctival swab sample was done as part of intramural research grant received from All India Institute of Medical Sciences, Rishikesh.
References
- Incidence and visual outcome of acute endophthalmitis after cataract surgery-the experience of an eye department in Scotland. Br J Ophthalmol. 2009;93:721-5.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of endophthalmitis after cataract surgery (2002-2008) at a Brazilian university hospital. Arq Bras Oftalmol. 2011;73:505-7.
- [CrossRef] [PubMed] [Google Scholar]
- Six-year incidence of endophthalmitis after cataract surgery: Swedish national study. J Cataract Refract Surg. 2013;39:15-21.
- [CrossRef] [PubMed] [Google Scholar]
- Role of external bacterial flora in the pathogenesis of acute postoperative endophthalmitis. Ophthalmology. 1991;98:639-49.
- [CrossRef] [PubMed] [Google Scholar]
- Identification and quantitation of conjunctival aerobic bacterial flora from healthy residents at different ages in Southwest China. Afr J Microbiol Res. 2011;5:192-7.
- [Google Scholar]
- Conjunctival bacterial flora and antibiotic resistance pattern in patients undergoing cataract surgery. Arq Bras Oftalmol. 2006;69:33-6.
- [CrossRef] [PubMed] [Google Scholar]
- Conjunctival anaerobic and aerobic bacterial flora in paediatric versus adult subjects. Br J Ophthalmol. 1988;72:448-51.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for endophthalmitis after cataract surgery: Predictors for causative organisms and visual outcomes. J Cataract Refract Surg. 2015;41:2410-6.
- [CrossRef] [PubMed] [Google Scholar]
- Postoperative endophthalmitis: A review of risk factors, prophylaxis, incidence, microbiology, treatment, and outcomes. Semin Ophthalmol. 2018;33:95-101.
- [CrossRef] [PubMed] [Google Scholar]
- Spectrum and susceptibilities of microbiologic isolates in the endophthalmitis vitrectomy study. Am J Ophthalmol. 1996;122:1-17.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence, clinical features, causative organisms, and visual outcomes of delayed-onset pseudophakic endophthalmitis. Eur J Ophthalmol. 2009;19:804-11.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment strategies and visual acuity outcomes in chronic postoperative propionibacterium acnes endophthalmitis. Ophthalmology. 1999;106:1665-70.
- [CrossRef] [PubMed] [Google Scholar]
- Delayed-onset pseudophakic endophthalmitis. Am J Ophthalmol. 1991;111:163-73.
- [CrossRef] [PubMed] [Google Scholar]
- Normal conjunctival flora as seen in adult patients at Kigali university Teaching Hospital. RMJ. 2013;70:22-4.
- [Google Scholar]
- Conjunctival bacterial flora and their antibiotic sensitivity among patients scheduled for cataract surgery in a tertiary hospital in south-east Nigeria. Graefes Arch Clin Exp Ophthalmol. 2021;259:443-8.
- [CrossRef] [PubMed] [Google Scholar]
- Conjunctival sac bacterial flora isolated prior to cataract surgery. Infect Drug Resist. 2012;5:37-41.
- [CrossRef] [PubMed] [Google Scholar]
- Aerobic bacterial flora of the normal conjunctiva at high altitude area of Shimla Hills in India: A hospital based study. Int J Ophthalmol. 2013;6:723-6.
- [Google Scholar]
- A study of the normal bacterial flora on the conjunctiva of patients undergoing cataract surgery to select the best pre-operative topical antibiotic. Int J A J Inst Med Sci. 2012;1:139-42.
- [Google Scholar]
- A study of normal bacterial flora of the conjunctiva in patients undergoing cataract surgery in a rural teaching hospital in RR district. J Sci Innov Res. 2014;3:164-7.
- [CrossRef] [Google Scholar]
- Conjunctival microflora and their antibiotic susceptibility in north Indians prior to cataract surgery. Int J Curr Microbiol Appl Sci. 2014;3:254-9.
- [Google Scholar]
- Bakterielle Keimbesiedelung der Konjunktiva mit Propionibacterium acnes vor und nach PolyvidonJod-Applikation vor intraokulären Eingriffen [Bacterial colonization of conjunctiva with Propionibacterium acnes before and after polyvidon iodine administration before intraocular interventions] Ophthalmologe. 1998;95:438-41.
- [CrossRef] [PubMed] [Google Scholar]
- The bacterial flora of the normal conjunctiva. Am J Ophthalmol. 1935;18:1114-6.
- [CrossRef] [Google Scholar]
- Association between ambient temperature, particulate air pollution and emergency room visits for conjunctivitis. BMC Ophthalmol. 2021;21:100.
- [CrossRef] [PubMed] [Google Scholar]
- Climatic influence on conjunctival bacteria of patients undergoing cataract surgery. Eye. 2004;18:778-84.
- [CrossRef] [PubMed] [Google Scholar]
- Characterization of the normal microbiota of the ocular surface. Exp Eye Res. 2013;117:99-105.
- [CrossRef] [PubMed] [Google Scholar]
- Eubacterial PCR for bacterial detection and identification in 100 acute postcataract surgery endophthalmitis. Invest Ophthalmol Vis Sci. 2008;49:1971-8.
- [CrossRef] [PubMed] [Google Scholar]
- Polybacterial community analysis in human conjunctiva through 16S rRNA gene libraries. Exp Eye Res. 2018;174:1-12.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of polybacterial communities in patients with postoperative, posttraumatic, and endogenous endophthalmitis through 16S rRNA gene libraries. J Clin Microbiol. 2014;52:1459-66.
- [CrossRef] [PubMed] [Google Scholar]






