Translate this page into:
Allergic aspergillosis in asthmatic patients in a tertiary hospital in the Kingdom of Bahrain
Address for correspondence: Dr. Abdullah A. Al-Saleh, Road 2904, Building 293, 3rd floor, Manama, Bahrain. E-mail: abdullahaas@agu.edu.bh
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
BACKGROUND INFORMATION:
Aspergillosis is an alarming complication in asthma, leading to worsening symptoms and irreversible lung damage. It is underdiagnosed among asthmatics worldwide, especially in our geographical region.
AIM:
This research was aimed to shed some light on the prevalence of Aspergillus sensitization and allergic bronchopulmonary aspergillosis (ABPA) in asthmatic patients in the Kingdom of Bahrain.
IMPORTANCE:
This project has never been conducted in the region and hopefully will lead to a better care for asthmatics.
METHODOLOGY:
Our study population consisted of adult outpatients visiting the pulmonary and chest diseases clinic at Al-Salmaniya Medical Complex (SMC); the main inclusion condition was manifesting respiratory distress symptoms related to one or more respiratory tract diseases. 152 individuals were selected; the study group consisted of 119 asthmatic patients manifesting respiratory distress. The control group consisted of 33 nonasthmatic patients that manifested pulmonary distress symptoms due to respiratory tract diseases other than asthma like chronic obstructive pulmonary disease, bronchitis, among others. Sera samples were collected in SMC and analyzed via Phadia-250 fluoro-enzyme-immunoassay to determine the levels of Aspergillus fumigatus-specific IgE. The rAsp f1 antigen was used against IgE.
RESULTS:
Our results indicated a 16% prevalence of A. fumigatus sensitization in asthmatics; also, 75.6% of asthmatics were sensitized to pollen grains, and 22.3% reported one or many food allergies. Furthermore, details of patients with significant levels of A. fumigatus- specific IgE were analyzed, and ABPA prevalence was estimated to be 10.1% in asthmatics.
CONCLUSION:
Increasing awareness toward these indolent diseases is required, as well as, more efforts in determining the burden of aspergillosis in other parts of the region.
Keywords
Allergic bronchopulmonary aspergillosis
aspergillosis
Aspergillus fumigatus
asthma
chronic pulmonary aspergillosis
Introduction
Aspergillus fumigatus is associated with most Aspergillus-related diseases which can be attributed to unique characteristics such as high dispersibility, minute conidial size, and the ability to sustain such size regardless of the degree of humidity, thus avoiding the mucociliary clearance and accessing the lower airways of the host.[1234]
A. fumigatus antigen sensitization is associated with airflow obstruction as well as severe asthma attacks.[56] Indeed, inhaling allergenic antigens might elicit hyperresponsiveness, but a crucial difference between A. fumigatus antigens and other allergens, say pollen or dust mites, is the ability to germinate and colonize the host.[7]
Among the wide spectrum of diseases caused by A. fumigatus, allergic bronchopulmonary aspergillosis (ABPA) stands as one of the most common; it is often defined as an indolent immunological respiratory disease, with potential progression, involving hyperresponsiveness reactions to persistent Aspergillus species (mostly A. fumigatus) in the airways.[89] Moreover, overlooked ABPA may progress to cause irreversible bronchi damage and lung fibrosis, as well as chronic sputum production, recurrent pneumonias, and ultimately respiratory failure; therefore, early diagnosis of ABPA is advised.[10]
Some disparities surround pinpointing the global incidence of ABPA in asthmatics, one report declared a 6% prevalence, whereas another analytical report concluded a 2.5% prevalence of ABPA in asthma.[1112] In countrywide projections, in New Zealand, ABPA was estimated to be 4.9%, in China was 2.5%, and in India, ABPA prevalence ranged from 6.9% to 22.3%.[61314151617]
Furthermore, among asthmatics, Aspergillus sensitization has been estimated to vary between 5.5% to 38.5% around the globe, according to ISHAM working group.[6131417181920]
Unfortunately, there are no reports in our region regarding Aspergillus sensitization and ABPA prevalence; therefore, the aim of this study is to determine the prevalence of A. fumigatus sensitization and ABPA in the Kingdom of Bahrain.
Methodology
This analytical case–control study was conducted in Al-Salmaniya Medical Complex, Kingdom of Bahrain where the relative humidity averages 47%–83% all year long.[21] Ethical approval was granted by the Research Technical Support Team at the Ministry of Health. All individuals signed a bilingual (Arabic and English) written consent before participating in the study as well as an overall verbal description of the study delivered by the principal investigator. Furthermore, the individuals responded to a questionnaire covering demographic characteristics, hypersensitivity profiles, and family history. Clinical examination of the respiratory system was performed by the corresponding physician at the clinic.
Participants’ characteristics
Male and female consecutive outpatients attending the pulmonary and chest diseases clinic were included in this study. The main inclusion condition was manifesting respiratory distress symptoms related to one or more respiratory tract diseases. Symptoms included coughing, wheezing, dyspnea, sputum production, chest pain and tightness, lethargy, and weight loss. The sample collection period spawned from April 23, 2017, to October 19, 2017.
152 adult patients, aged 18 years and above, were selected. The study group consisted of 119 asthmatic patients manifesting respiratory distress. According to the patient's medical record, the Global Initiative for Asthma guidelines,[22] and the attending physician characterization, the patient was labeled as asthmatic and included in the study group.
The control group consisted of 33 nonasthmatic patients that manifested pulmonary distress symptoms due to respiratory tract diseases other than asthma such as chronic obstructive pulmonary disease, sinusitis, interstitial lung disease, bronchiectasis, bronchitis, pneumonia, tuberculosis, emphysema, nonspecific interstitial pneumonia, and lung cancer among others. The attending physician provided proper characterization for the patient's ailment and the corresponding symptoms, as well as consulting the patient's medical record.
Patients who were diagnosed with aspergillosis before participating in the study were excluded to avoid selection bias.
Additional medical information such as total serum IgE level, radiographic imaging, and related diseases were obtained from the patient's medical profile after the completion of the sample collection period, compensating for any missing details.
Immunological analysis
Venipuncture apparatus was used to collect blood, in accordance with Henry's Clinical Diagnosis and Management by Laboratory Methods[23] via a needle/adapter assembly attached to a test tube with plastic/rubber stopper. The tubes used in this study were gold-capped BD Vacutainer serum separation tube (SST II advance) ref: 367955; these tubes included silica clot activator separation gel mainly used for serum separation. The tube was allowed to sit for about 30 min to allow coagulation, and centrifuging was performed for 10 min at 8000 rpm. There was no need to transfer the serum to 5 ml glass tubes for preservation because the separation gel in the tubes acted as a physical barrier between the clot and the serum. The tubes were then stored at −80°C freezer until the time of serological analysis.
Serological analysis was carried out on Phadia 250 ImmunoCap (Phadia, Uppsala, Sweden) in determining the quantitative values of total serum IgE and A. fumigatus-specific IgE according to the user manual.[24]
Total serum IgE
Values of >417 kU/L were considered positive. This test was specifically requested in case it was missing from the medical record of the patient.
Aspergillus Fumigatus-specific IgE
The antigen used against the specific IgE was the recombinant protein rAsp f1 that shows a high degree of homology with ribotoxins isolated from A. fumigatus. Values more than 0.35 kU/L were considered positive.
Aspergillus immediate sensitization was defined by the presence of A. fumigatus-specific IgE >0.35 kU/L.
Radiographic analysis
Radiographic evidence was not always available because our population consisted of asthmatic outpatients attending the pulmonary clinic and were not managed as aspergillosis patients; incidentally, they were not administered a computed tomography (CT) scan by the corresponding clinician. Nonetheless, we were able to acquire high-resolution CT scans from the medical records of some patients.
All scans were reviewed by pulmonary radiologists and specialists in pulmonary diseases. The diagnostic features for ABPA included bronchiectasis, mucous plugging, parenchymal scarring, and consolidation.
Allergic bronchopulmonary aspergillosis criteria
The diagnosis of ABPA followed the minimal criteria proposed by Greenberger[10] that included
-
Asthma
-
Immediate sensitization to A. fumigatus
-
Elevated level of Total serum IgE >417 kU/L
-
Elevated A. fumigatus-specific IgE >0.35 kU/L
-
Proximal bronchiectasis.
In case of Sero-ABPA, bronchiectasis is not required.
Statistical analysis
Data were recorded and analyzed via IBM SPSS statistics Version 20 for Windows. Armonk, NY: IBM Corp, Released 2011.
Independent sample t-test was utilized to compare the mean levels of A. fumigatus-specific IgE between asthmatic and nonasthmatic individuals.
The prevalence was calculated following the formula below:
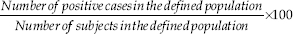
The odds ratio (OR) and 95% confidence interval (CI) values were used to assess the strength of association between the onset of asthma and various medical characteristics such as symptoms and hypersensitivity profile; calculations were through binary logistic regression.
For all statistical analysis, results with P < 0.05 were considered statistically significant.
Results
During this analytical case–control study, 172 outpatients were selected, 20 of which were rejected due to sampling or storing errors, leaving 152 eligible participants (119 asthmatics and 33 controls).
Participants’ characteristics
In this study, 97.5% of participants were Bahrainis; moreover, females comprised most of the study population, a bulk of 71% compared to 29% for males, as shown in Table 1. Furthermore, the ages of the participants were in the range of 18–92, averaging about 54 years of age; the vast majority of the participants were in the wide range of 26 to >66 but a solid peak substantiates at the 46–65 age group in both asthmatic (35%) and nonasthmatic (11.2%) patients.
| General features | Population, n (%) | Asthmatic % | Nonasthmatic % |
|---|---|---|---|
| Age (18-92, average=54.3) (years) | |||
| 18-25 | 6 (3.8) | 3.2 | 0.6 |
| 26-45 | 35 (23) | 21 | 2 |
| 46-65 | 70 (46.2) | 35 | 11.2 |
| 66+ | 41 (27) | 19 | 8 |
| Gender | |||
| Male | 44 (29) | 19 | 10 |
| Female | 108 (71) | 59 | 12 |
| Nationality | |||
| Bahraini | 148 (97.5) | 75.8 | 21.7 |
| Others | 4 (2.5) | 2.5 | 0 |
| Smoking | |||
| Yes | 31 (21) | 13 | 8 |
| No | 118 (79) | 65.6 | 13.4 |
| Indoor pets | |||
| Yes | 36 (26) | 19 | 7 |
| No | 107 (74) | 60 | 14 |
| Childhood asthma | |||
| Yes | 18 (12) | 12 | 0 |
| No | 132 (88) | 67.3 | 20.7 |
| Nebulizer support | |||
| Occasional | 56 (37.7) | 33 | 4.7 |
| Regular | 23 (15.5) | 12.1 | 3.4 |
| No | 69 (46.8) | 34 | 12.8 |
| Oxygen support | |||
| Yes | 7 (4.8) | 3.4 | 1.4 |
| No | 140 (95.2) | 75.5 | 19.7 |
The majority of the individuals were atopic, about 89.5% of the population, and 96.6% of asthmatic individuals (OR = 13.7, 95% CI = 3.9–47.8, P < 0.001). Our data in Table 2 shows occurrence of conditions such as eczema (41.4%), allergic rhinitis (61.2%), and allergic conjunctivitis (48.7%), many of which coincided with asthma. Moreover, sensitization reactions in asthmatic individuals to pollen grains, dust, smoke, and fumes were reported, amounting to rates such as 75.6%, 92.4%, and 73.1%, respectively (P < 0.05). In addition, the most commonly reported form of food allergy was allergy to seafood (6%) and eggs (4.2%), reported only by asthmatic participants; while a whopping 83% of the population experienced no food allergy (79% of asthmatics, 97% of nonasthmatics; OR = 8.5, 95% CI = 1.1–65.3, P = 0.0395).
| Hypersensitivity profile | Population (n=152), n (%) | Asthmatic (n=119), n (%) | Control (n=33), n (%) | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| Atopy | 136 (89.5) | 115 (96.6) | 21 (63.6) | <0.001 | 13.7 | 3.9-47.8 |
| Eczema | 63 (41.4) | 58 (48.7) | 5 (15.2) | 0.006 | 3.8 | 1.5-10 |
| Allergic rhinitis | 93 (61.2) | 78 (65.5) | 15 (45.5) | 0.184 | 1.7 | 0.77-3.8 |
| Conjunctivitis | 74 (48.7) | 67 (56.3) | 7 (21.2) | 0.005 | 3.6 | 1.5-8.6 |
| Pollen sensitization | 106 (69.7) | 90 (75.6) | 16 (48.5) | 0.002 | 3.7 | 1.6-8.5 |
| Dust sensitization | 128 (84.2) | 110 (92.4) | 18 (54.5) | <0.001 | 6.9 | 2.6-18.2 |
| Fumes sensitization | 102 (67.1) | 87 (73.1) | 15 (45.5) | 0.007 | 2.98 | 1.3-6.7 |
| Food allergy | 26 (17.1) | 25 (21) | 1 (3) | 0.0395 | 8.5 | 1.1-65.3 |
| Egg | 5 (3.3) | 5 (4.2) | 0 | |||
| Seafood | 7 (4.6) | 7 (6) | 0 | |||
| Chicken | 2 (1.3) | 2 (1.7) | 0 | |||
| Mango | 3 (2) | 3 (2.5) | 0 | |||
| Others | 5 (3.3) | 5 (4.2) | 0 |
OR=Odds ratio, CI=Confidence interval
As shown in Table 3, the test population reported experiencing symptoms such as coughing (93.3%; OR = 1.56, 95% CI = 1.04–2.33, P = 0.03), wheezing (78.2%; OR = 5.29, 95% CI = 2.23–12.56, P < 0.001), and night distress (39.5%; OR = 3.29, 95% CI = 1.12-9.72, P = 0.031); many of these individuals were in the asthmatic group of patients.
| Symptoms | Population (n=152), n (%) | Asthmatic (n=119), n (%) | Control (n=33), n (%) | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| Cough | 142 (93.3) | 112 (94.1) | 30 (91) | 0.030 | 1.56 | 1.04-2.33 |
| Wheezing | 119 (78.2) | 102 (85.7) | 17 (51.5) | <0.001 | 5.29 | 2.23-12.56 |
| Sputum production | 113 (74.3) | 92 (77.3) | 21 (63.6) | 0.121 | 1.83 | 0.85-3.93 |
| Dyspnea | 97 (63.5) | 78 (65.5) | 19 (57.6) | 0.963 | 1.03 | 0.34-3.09 |
| Chest tightness | 66 (43.4) | 55 (46.2) | 11 (33.3) | 0.344 | 1.57 | 0.62-4.01 |
| Chest pain | 57 (37.6) | 48 (40.3) | 9 (27.3) | 0.255 | 1.73 | 0.67-4.47 |
| Lethargy | 48 (31.5) | 41 (34.5) | 7 (21.2) | 0.232 | 2.05 | 0.63-6.65 |
| Hemoptysis | 5 (3.3) | 3 (2.5) | 2 (6.1) | 0.499 | 0.59 | 0.13-2.71 |
| Night distress | 60 (39.5) | 53 (44.5) | 7 (21.2) | 0.031 | 3.29 | 1.12-9.72 |
| Morning distress | 37 (24.4) | 33 (27.7) | 4 (12.1) | 0.079 | 2.98 | 0.88-10.05 |
| Fever | 45 (29.6) | 38 (32) | 7 (21.2) | 0.585 | 1.27 | 0.54-3.03 |
| Weight loss | 18 (12) | 9 (7.6) | 9 (27.3) | 0.001 | 0.13 | 0.04-0.44 |
| Vertigo | 8 (5.3) | 5 (4.2) | 3 (9.1) | 0.277 | 0.44 | 0.1-1.93 |
OR=Odds ratio, CI=Confidence interval
According to Table 4, the most commonly reported chronic diseases were diabetes (23.1%) and hypertension (27%). Moreover, 10% of our population developed bronchiectasis, majorly nonasthmatic individuals (27.2%; OR = 0.14, 95% CI = 0.05–0.45, P = 0.001).
| Common underlying conditions | Population (n=152), n (%) | Asthmatic (n=119), n (%) | Control (n=33), n (%) | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| Bronchiectasis | 15 (10) | 6 (5) | 9 (27.2) | 0.001 | 0.14 | 0.05-0.43 |
| Old tuberculosis | 2 (1.3) | 2 (1.7) | 0 | |||
| COPD | 10 (6.6) | 0 | 10 (30.3) | |||
| Lung cancer | 2 (1.3) | 2 (1.7) | 0 | |||
| Pneumonia | 6 (4) | 3 (2.5) | 3 (9.1) | 0.104 | 0.25 | 0.05-1.33 |
| Diabetes | 35 (23.1) | 28 (23.5) | 7 (21.2) | 0.779 | 1.14 | 0.45-2.91 |
| Hypertension | 41 (27) | 31 (26) | 10 (30.3) | 0.598 | 0.79 | 0.34-1.87 |
| GERD | 17 (11.2) | 13 (11) | 4 (12.1) | 0.193 | 0.88 | 0.27-2.93 |
| Osteoporosis | 5 (3.4) | 4 (3.4) | 1 (3) | 0.925 | 1.11 | 0.12-10.3 |
| Sinusitis | 7 (4.7) | 4 (3.4) | 3 (9.1) | 0.182 | 0.35 | 0.07-1.63 |
COPD=Chronic obstructive pulmonary disease, GERD=Gastro-oesophageal reflux disease, OR=Odds ratio, CI=Confidence interval
Allergic bronchopulmonary aspergillosis-positive individuals
According to Table 5, 21 patients (13.8%) showed positive levels of specific IgE against A. fumigatus, 13 females and 8 males; ages ranged from 30 to 88, averaging 55.6 years of age. 19 individuals were asthmatic; furthermore, 12 gave significant levels of total serum IgE (>417 kU/L) surpassing the designated positive cutoff. Also, 4 patients were administered systemic corticosteroid therapy at the time of sample collection, and radiological evidence was available for 7 individuals only, the missing ones were not requested by the corresponding physician.
| Individual number | Gender | Age | Asthma | Corticosteroid therapy | Total IgE levels (kU/L) | Af sIgE levels | Radiological findings |
|---|---|---|---|---|---|---|---|
| 1 | Female | 56 | Yes | - | >417 | Positive | Missing |
| 2 | Female | 68 | Yes | - | >417 | Positive | Bronchiectasis and fibrosis |
| 3 | Male | 77 | Yes | - | <417 | Positive | Bronchiectasis and fibrosis |
| 4 | Female | 88 | Yes | - | >417 | Positive | Bronchiectasis and fibrosis |
| 5 | Male | 38 | Yes | - | >417 | Positive | Missing |
| 6 | Female | 59 | Yes | Yes | >417 | Positive | Missing |
| 7 | Male | 55 | Yes | - | >417 | Positive | Bronchiectasis and mucous |
| plugs | |||||||
| 8 | Male | 49 | Yes | - | >417 | Positive | Normal |
| 9 | Female | 34 | Yes | Yes | >417 | Positive | Bronchiectasis |
| 10 | Female | 66 | Yes | Yes | >417 | Positive | Missing |
| 11 | Female | 41 | Yes | - | >417 | Positive | Missing |
| 12 | Male | 64 | No | - | >417 | Positive | Bronchiectasis and fibrosis |
| 13 | Female | 30 | Yes | - | >417 | Positive | Missing |
| 14 | Male | 74 | Yes | - | <417 | Positive | Missing |
| 15 | Female | 55 | Yes | Yes | <417 | Positive | Missing |
| 16 | Male | 65 | Yes | - | <417 | Positive | Missing |
| 17 | Female | 48 | No | - | <417 | Positive | Missing |
| 18 | Female | 65 | Yes | - | <417 | Positive | Missing |
| 19 | Female | 39 | Yes | - | <417 | Positive | Missing |
| 20 | Female | 46 | Yes | - | <417 | Positive | Missing |
| 21 | Male | 50 | Yes | - | <417 | Positive | Missing |
Af sIgE levels=Aspergillus fumigatus-specific IgE levels, IgE=Immunoglobulin E
Moreover, 5 individuals fulfilled the ABPA criteria whereas 7 only missed the radiological evidence portion of the criteria, which categorizes them as “Sero-ABPA positive.” Therefore, ABPA prevalence in our study is about (12/119) 10.1% in asthmatic patients.
The significance of Aspergillus Fumigatus-specific IgE
Independent sample t-test was conducted to determine a significant difference in the levels of specific immunoglobulins between asthmatic and nonasthmatic individuals, as shown in Table 6. Moreover, 19 asthmatic individuals and 2 controls showed elevated levels of A. fumigatus-specific IgE, beyond the designated cutoff at 0.35 kU/L. With 95% confidence, P < 0.05 meaning that there was a significant difference in the means of IgE levels between asthmatics and controls.
| Immunoglobulin | Asthmatic (n=119) | Control (n=33) | Mean difference | P | 95% CI | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Positives (%) | Mean | SD | Positives (%) | ||||
| Af sIgE | 0.22 | 0.20 | 19 (16) | 0.13 | 0.09 | 2 (6.1) | 0.09 | 0.01 | 0.02-0.16 |
SD=Standard deviation, CI=Confidence interval, Af sIgE=Aspergillus fumigatus-specific IgE, IgE=Immunoglobulin E
Discussion
Substantial evidence illuminates a viable relation between Aspergillus sensitization and worsening asthma severity, in young children to aging adults.[78] Many reasons might amount to that correlation such as the persistent production of thick bronchial secretions in some asthmatics, that accommodates a hospitable growth environment for fungi; another reason might be genetic predisposition, for example, in patients with a defect in surfactant A2.[2526] Among asthmatics, Aspergillus sensitization has been estimated to vary between 5.5% to 38.5% around the globe, according to ISHAM working group.[6131417181920] Comparatively, our findings show that 19 of 119 asthmatic patients exhibited A. fumigatus sensitization, giving a 16% prevalence of A. fumigatus sensitization in asthmatic patients, supported with significant statistical correlation at 95% confidence, P < 0.05. This middle range prevalence seems to be lower than the regionally closest recorded rate by Al-Mobeireek et al.[18] at 23% although the Saudi study in that particular area included smaller sample size at 54 patients. Furthermore, they used skin prick test which lacks accuracy in comparison with Phadia 250 ImmunoCap fluoro-enzyme immunoassay.[2728]
In this study, atopy was reported by 97% of asthmatic individuals (P < 0.05, OR = 13.7, 95% CI = 3.9–47.8); however, lower rates were reported in New Zealand, India, Singapore, Europe, and United States, at 86.8% 75%, 78%, 72%, and 62.1%, respectively.[613202930] Moreover, allergic rhinitis in asthma was reported in a range of 63–85%; our findings suggest a 65% occurrence of allergic rhinitis in asthmatics.[620] It is worth noting that 95% of asthmatics are polysensitized to various allergens.[831] Our findings show that 75.6% of asthmatics were sensitized to pollen grains (P < 0.05, OR = 3.7, 95% CI = 1.6–8.5), and 22.3% reported one or many food allergies (P < 0.05, OR = 8.3, 95% CI = 1.08–64.1). In the literature, sensitization to tree and grass pollen was estimated to be 49% in asthmatics; whereas, food allergies in adult asthmatics was a diminutive 2.5%.[2932] The discrepancies between our results and the reports in the literature can be attributed to many reasons, one of which is that the data used to construct our estimations were reported by the individuals themselves with the occasional support of the medical profile whenever applicable. Another reason is that differences in the population size and characteristics would most likely produce deviations. Furthermore, the dearth of reports in some research topics, like food allergy in asthmatic adults, hindered objective comparisons.
In addition, asthmatic candidates reported experiencing cough (94.1%; P < 0.05, OR = 1.56, 95% CI = 1.04–2.33), wheeze (85.7%; P < 0.05, OR = 5.29, 95% CI = 2.23–12.56), dyspnea (65.5%), sputum production (77.3%), and night distress (44.5%; P < 0.05, OR = 3.29, 95% CI = 1.12–9.72); these rates were similar to the estimates in the studies conducted in Saudi Arabia and India.[1827] Uncharacteristically, hemoptysis was reported in 3 (2.5%) asthmatic individuals that were not diagnosed with aspergillosis or other pulmonary condition; so, further analysis needs to be implemented.
Furthermore, the continuous exposure to Aspergillus antigens will consequently lead to pulmonary aspergillosis (e.g., ABPA and CPA), causing liable severe asthma, lung tissue destruction, respiratory failure, and eventually death.[533] ABPA is a potentially progressive pulmonary disease that is caused by a complex hyperreactivity response to Aspergillus antigens in the lungs.[9] Some disparities surround pinpointing the incidence of ABPA in asthmatics; one report declared a 6% prevalence whereas another analytical report concluded a 2.5% prevalence of ABPA in asthma.[1112] Moreover, countrywide projections provided diverse rates, in New Zealand, ABPA was estimated to be 4.9%; in China, it was 2.5%; and in India, ABPA prevalence ranged from 6.9% to 22.3%.[61314151617] Conjunctively, our findings suggest a 10.1% prevalence of ABPA in asthmatics.
One of the main causes of the disparity in ABPA prevalence is the absence of consensus on the parameters of ABPA diagnostic criteria.[34] Indeed, most of the cases reflect the corresponding specialist opinion, which might be delayed or incomplete; also, candidates at different ABPA stages might not fulfill all the diagnostic parameters, which deems ABPA prevalence as a speculative manner prone to disparity and error.[611]
It has been proposed by Greenberger[10] that ABPA should be considered in any asthmatic patient who shows any of the following signs: bronchiectasis, history of pneumonias, mucous plugs, elevated A. fumigatus-specific antibodies, and increased severity of asthma symptoms; Henceforth, proper case management should be administered. Moreover, oversighting ABPA diagnosis might occur when negative radiographic imaging is obtained, thus awaiting the manifestation of bronchiectasis to secure the diagnosis despite the immunological evidence; this type of clinical mishap overlooks the early stages of ABPA the “Sero-ABPA”; evidently, underdiagnosing the disease and decreasing the reported prevalence.[1035] Therefore, in our study, it was crucial to avoid this mishap, thereupon, accounting the immunological evidence as the quasi-main evidence of ABPA along with symptoms’ manifestation, then supplementing the radiographic evidence whenever possible. Unfortunately, radiographic evidence was not always available because our population consisted of asthmatic outpatients attending the pulmonary clinic and were not managed as aspergillosis patients; incidentally, they were not administered a CT-scan by the corresponding clinician. This measure of patient selection provided benefits like minimizing inclusion bias, although missed some evidence that cannot be determined as positive or negative. Nevertheless, some studies did not require central bronchiectasis to diagnose ABPA in asthmatic patients, relying mostly on immunological evidence, so our characterization of ABPA was not a novel approach but a tested measurement.[3637] All in all, 5 out of 119 (4.2%) asthmatic patients have fulfilled the designated ABPA criteria, whereas additional 7 (5.9%) only missed the radiographic evidence; therefore, the combined prevalence of ABPA in asthmatic patients in Bahrain is 10.1%.
Furthermore, risk factors facilitating the manifestation and development of aspergillosis are many, one of which is bronchiectasis in 73% of ABPA patients; yet, among asthmatics, lower prevalence was recorded at 15.5%, although our findings only reported 5% prevalence (P < 0.05, OR = 0.14, 95% CI = 0.05–0.43).[1537] In addition, tuberculosis and lung cancer are of the most critical risk factors in developing aspergillosis, reaching a prevalence of 81% and 10.3%, respectively; yet, in our asthmatic group, only 2% were affected by each condition.[3839] Interestingly, diabetes affected about 12% of aspergillosis patients, although a higher prevalence was reported in our findings at 23.5% of asthmatics. Viable reason can explain such differences between our findings and other articles, which is that most studies were conducted retrospectively, giving the option to include the most characteristic subject in the manner of the research, contrary to our prospective research.
Despite the lack of consensus in the parameters of ABPA criteria, elevated levels of Aspergillus-specific IgE are unanimously considered an essential part in the diagnosis.[40] In fact, it is currently considered the most sensitive screening tool for ABPA in asthmatics, surpassing skin prick test and sputum cultures, and providing a 100% sensitivity and 77.3% specificity.[2740] Unfortunately, the absence of standardized antigens is a major drawback for this test; generally, the desired results should administer a genuine, species-specific indication of sensitization rather than levels inflated with cross-reactivity.[41]
It is worth noting that most fungal allergens in varied species belong to the same protein families (e.g., MnSOD, thioredoxin, and cyclophilin) and share similarities in the three-dimensional structure and charge distribution; hence, cross-reactivity is eminent while using crude fungal extracts, as it has been the case for many years.[42] Therefore, it is important to rely on an antigen that is confined in a particular species, as well as, lacks homologies in the genome of said species, hence avoiding suspicion of crossreactivity with any similar protein to our designated antigen.[43]
Moreover, few A. fumigatus antigens fulfill these conditions, one of which is Asp f1 that we utilized to measure the levels of specific IgE in the serum, it represents a species-specific protein as well as no recorded homology with any other fungus and among other Aspergillus species.[4243] Furthermore, Asp f1 is an 18 kD A. fumigatus-specific allergen that belongs to the mitogillin family of cytotoxins.[44] It is not generated in the conidia but produced in the mycelium within 12 h of germination; and while exposure to A. fumigatus is common, minute number of people immunologically project its presence, yet it is found in 75%–85% of ABPA patients; therefore, Asp f1 is a viable marker for aspergillosis.[4445] Moreover, Asp f1 induces T-cell proliferation, inhibits protein synthesis, and ultimately contributes to the pathogenesis of aspergillosis, especially during the early stages of the disease.[46] In related Japanese studies,[4047] they stated that Asp f1 showed a good diagnostic performance, and it can effectively characterize ABPA as well as provide genuine indication of A. fumigatus sensitization; also, they estimated a 77% sensitivity and 85% specificity for Asp f1, surpassing many of the available screening tools.
Conclusion and Limitations
In this research, valuable insight was attained on the effect of Aspergillus exposure on asthmatic patients. For the first time in the Kingdom of Bahrain, the prevalence of Aspergillus sensitization and ABPA in asthmatic patients were determined (16% and 10.1%, respectively), by analyzing various immunological markers such as A. fumigatus-specific IgE, with the support of clinical and radiological evidences.
Such findings will hopefully lead to a better management of asthmatic patients and enhance our understanding of Aspergillus epidemiology in the region.
One immense limitation is not utilizing more than one antigen for the serological analysis, we could not mitigate this limitation because of the budget ceiling that forced us to choose between a sizable population or using various antigens, and given that aspergillosis is not very common, we opted to include as many individuals as possible. Furthermore, the lack of regional and global reports left few knowledge blanks about the topic.
Financial support and sponsorship
This research was sponsored and funded by the Ministry of Civil Services in The State of Kuwait.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank all the contributing technicians and specialists in the blood collection laboratory and HLA laboratory at SMC, as well as the microbiology laboratory specialists at AGU, especially, Mr. Ali Al-Mahmeed. Also, we like to address our gratitude for Dr. Ahmad Jaradat for helping in the statistical analysis.
References
- Pathogenesis of Aspergillus fumigatus in invasive aspergillosis. Clin Microbiol Rev. 2009;22:447-65.
- [Google Scholar]
- Genes and molecules involved in Aspergillus fumigatus virulence. Rev Iberoam Micol. 2005;22:1-23.
- [Google Scholar]
- Aspergillus fumigatus – What makes the species a ubiquitous human fungal pathogen? PLoS Pathog. 2013;9:e1003743.
- [Google Scholar]
- Effect of relative humidity on the aerodynamic diameter and respiratory deposition of fungal spores. Atmos Environ. 1996;30:3967-74.
- [Google Scholar]
- A comparison of the prevalence of sensitization to Aspergillus antigens among asthmatics in cleveland and london. J Allergy Clin Immunol. 1978;62:9-14.
- [Google Scholar]
- Sensitization to Aspergillus antigens and occurrence of allergic bronchopulmonary aspergillosis in patients with asthma. Chest. 2005;127:1252-9.
- [Google Scholar]
- The link between fungi and severe asthma: A summary of the evidence. Eur Respir J. 2006;27:615-26.
- [Google Scholar]
- Abridged version of the AWMF guideline for the medical clinical diagnostics of indoor mould exposure: S2K guideline of the German Society of Hygiene, Environmental Medicine and Preventive Medicine (GHUP) in collaboration with the German Association of Allergists (AeDA), the German Society of Dermatology (DDG), the German Society for Allergology and Clinical Immunology (DGAKI), the German Society for Occupational and Environmental Medicine (DGAUM), the German Society for Hospital Hygiene (DGKH), the German Society for Pneumology and Respiratory Medicine (DGP), the German Mycological Society (DMykG), the Society for Pediatric Allergology and Environmental Medicine (GPA), the German Federal Association of Pediatric Pneumology (BAPP), and the Austrian Society for Medical Mycology (ÖGMM) Allergo J Int. 2017;26:168-93.
- [Google Scholar]
- When to suspect and work up allergic bronchopulmonary aspergillosis. Ann Allergy Asthma Immunol. 2013;111:1-4.
- [Google Scholar]
- Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication chronic pulmonary aspergillosis in adults. Med Mycol. 2013;51:361-70.
- [Google Scholar]
- Allergic bronchopulmonary aspergillosis in the asthma clinic. A prospective evaluation of CT in the diagnostic algorithm. Chest. 2000;118:66-72.
- [Google Scholar]
- Prevalence of allergic bronchopulmonary aspergillosis in Chinese patients with bronchial asthma. Zhonghua Jie He He Hu Xi Za Zhi. 2011;34:909-13.
- [Google Scholar]
- Prevalence of allergic bronchopulmonary aspergillosis in patients with bronchial asthma. Asian Pac J Allergy Immunol. 2000;18:181-5.
- [Google Scholar]
- Clinical significance of decline in serum IgE levels in allergic bronchopulmonary aspergillosis. Respir Med. 2010;104:204-10.
- [Google Scholar]
- Aspergillus hypersensitivity and allergic bronchopulmonary aspergillosis among asthma patients in Eastern India. J Indian Med Assoc. 2010;108:863-5.
- [Google Scholar]
- Allergic bronchopulmonary mycosis in patients with asthma: Period prevalence at a University hospital in Saudi Arabia. Respir Med. 2001;95:341-7.
- [Google Scholar]
- Clinical significance of hyperattenuating mucoid impaction in allergic bronchopulmonary aspergillosis: An analysis of 155 patients. Chest. 2007;132:1183-90.
- [Google Scholar]
- Sensitization to Aspergillus species is associated with frequent exacerbations in severe asthma. J Asthma Allergy. 2017;10:131-40.
- [Google Scholar]
- GINA. Global Strategy for Asthma Management and Prevention. 2017. Available from www.ginasthma.org
- [Google Scholar]
- Henry's clinical diagnosis and management by laboratory methods. (22nd ed). Philadelphia, PA: Elsevier/Saunders; 2011.
- [Google Scholar]
- Phadia 250 User Manual. Sweden: Phadia AB; 2010.
- Association of polymorphisms in the collagen region of SP-A2 with increased levels of total IgE antibodies and eosinophilia in patients with allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol. 2003;111:1001-7.
- [Google Scholar]
- Allergic Aspergillus sinusitis and its association with allergic bronchopulmonary aspergillosis. Asia Pac Allergy. 2011;1:130-7.
- [Google Scholar]
- Diagnostic performance of various tests and criteria employed in allergic bronchopulmonary aspergillosis: A latent class analysis. PLoS One. 2013;8:e61105.
- [Google Scholar]
- Diagnostic accuracy of skin-prick testing for allergic rhinitis: A systematic review and meta-analysis. Allergy Asthma Clin Immunol. 2016;12:20.
- [Google Scholar]
- Population-based study of multiplexed IgE sensitization in relation to asthma, exhaled nitric oxide, and bronchial responsiveness. J Allergy Clin Immunol. 2012;130:397-402.e2.
- [Google Scholar]
- Total IgE levels and asthma prevalence in the US population: Results from the national health and nutrition examination survey 2005-2006. J Allergy Clin Immunol. 2009;124:447-53.
- [Google Scholar]
- Placebo-controlled double-blind food challenge in asthma. J Allergy Clin Immunol. 1986;78:1139-46.
- [Google Scholar]
- Allergic bronchopulmonary aspergillosis. Natural history and classification of early disease by serologic and roentgenographic studies. Arch Intern Med. 1986;146:916-8.
- [Google Scholar]
- Aspergillus hypersensitivity and allergic bronchopulmonary aspergillosis in patients with bronchial asthma: Systematic review and meta-analysis. Int J Tuberc Lung Dis. 2009;13:936-44.
- [Google Scholar]
- Serologic allergic bronchopulmonary aspergillosis (ABPA-S): Long-term outcomes. Respir Med. 2012;106:942-7.
- [Google Scholar]
- Development of chronic pulmonary aspergillosis in adult asthmatics with ABPA. Respir Med. 2015;109:1509-15.
- [Google Scholar]
- Allergic bronchopulmonary aspergillosis: Lessons from 126 patients attending a chest clinic in North India. Chest. 2006;130:442-8.
- [Google Scholar]
- Clinical manifestations and treatment outcomes of pulmonary aspergilloma. Korean J Intern Med. 2004;19:38-42.
- [Google Scholar]
- Underlying conditions in chronic pulmonary aspergillosis including simple aspergilloma. Eur Respir J. 2011;37:865-72.
- [Google Scholar]
- Serological diagnosis of allergic bronchopulmonary mycosis: Progress and challenges. Allergol Int. 2016;65:30-6.
- [Google Scholar]
- Selected recombinant Aspergillus fumigatus allergens bind specifically to IgE in ABPA. Clin Exp Allergy. 2000;30:988-93.
- [Google Scholar]
- Cross-reactivity among fungal allergens: A clinically relevant phenomenon? Mycoses. 2009;52:99-106.
- [Google Scholar]
- Genomic analysis of allergen genes in Aspergillus spp: The relevance of genomics to everyday research. Med Mycol. 2007;45:17-26.
- [Google Scholar]
- Environmental exposure to Aspergillus fumigatus allergen (Asp f I) Clin Exp Allergy. 1993;23:326-31.
- [Google Scholar]
- Use of a synthetic peptide epitope of asp f 1, a major allergen or antigen of Aspergillus fumigatus, for improved immunodiagnosis of allergic bronchopulmonary aspergillosis. Clin Diagn Lab Immunol. 2004;11:552-8.
- [Google Scholar]
- Aspergillus ribotoxins react with IgE and IgG antibodies of patients with allergic bronchopulmonary aspergillosis. J Lab Clin Med. 1994;123:749-56.
- [Google Scholar]
- Molecular-based allergy diagnosis of allergic bronchopulmonary aspergillosis in Aspergillus fumigatus-sensitized Japanese patients. Clin Exp Allergy. 2015;45:1790-800.
- [Google Scholar]





