Translate this page into:
Comparative evaluation of galactomannan test with bronchoalveolar lavage and serum for the diagnosis of invasive aspergillosis in patients with hematological malignancies
Address for correspondence: Dr. Malini R. Capoor, Department of Microbiology, Vardhman Mahavir Medical College and Safdarjung Hospital, New Delhi - 110 029, India. E-mail: rajeevmalini@rediffmail.com
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
INTRODUCTION:
Invasive pulmonary aspergillosis (IPA) is a major cause of morbidity and mortality in patients with hematological malignancies. In recent years, testing for values of galactomannan (GM) in serum and bronchoalveolar lavage (BAL) fluid has been investigated as a diagnostic test for IPA for such patients, but global experience and consensus on optical density (OD) cutoffs, especially for BAL galactomannan remains lacking.
METHODS:
We performed a prospective case–control study to determine an optimal BAL GM OD cutoff for IPA in at-risk patients. Cases were subjects with hematological diagnoses who met established revised definitions for proven or probable IPA established by the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group (EORTC/MSG, 2008), without the use of BAL GM results. Exclusion criteria included the use of piperacillin/tazobactam and use of antifungals that were active against Aspergillus spp. before bronchoscopy. There were two control groups: patients with hematological diagnoses not meeting definitions for proven or probable IPA and patients with nonhematological diagnoses with no evidence of aspergillosis. Following bronchoscopy and BAL, GM testing was performed using the Platelia Aspergillus seroassay in accordance with the manufacturer's instructions.
RESULTS:
There were 51 cases and 20 controls. Cases had higher BAL fluid GM OD indices (ODIs) (mean: 1.27 and range: 0.4–3.78) compared with controls (mean: 0.26 and range: 0.09–0.35). Receiver operating characteristic analysis demonstrated an optimum ODI cutoff of 1.0, with high specificity (100%) and sensitivity (87.5%) for diagnosing IPA.
CONCLUSIONS:
Our results support BAL GM testing as a reasonably safe test with higher sensitivity compared to serum GM testing in at-risk patients with hematological diseases. A higher OD cutoff is necessary to avoid overdiagnosis of IPA.
Keywords
Bronchoalveolar lavage
galactomannan
hematological malignancies
serum
Introduction
Aspergillus species and its spores are ubiquitously distributed in the environment. They are responsible for invasive pulmonary aspergillosis (IPA), a serious clinical form of disease in immunocompromised patients.[1] The major risk groups include patients with hematological malignancy, acute leukemia, solid tumors, solid organ, and hematopoietic stem cell transplant recipients and with defective neutrophil function.[234] If the mycotic infection is diagnosed accurately and in time, it will allow the clinician to initiate antifungal therapy with the most appropriate drug. Usually, diagnosis of IPA depends on a constellation of clinical, radiological, and microbiological criteria. There is an unmet need to explore nonculture-based diagnostic tests including detection of circulating antigens, for example, galactomannan (GM), β-glucan, and other polymerase chain reaction (PCR)-based nucleic acid detection tests. Aspergillus species have angioinvasive properties. During the process of tissue invasion by fungal hyphae, a heat stable polysaccharide component of fungal cell wall called GM is released. Its ability to be detected in body fluids and its appearance in blood in the early stages even before clinical or radiological signs makes it an ideal candidate for the diagnosis of IPA.[5] Detection of GM antigen has been found adequate in European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycosis Study Group (EORTC/MSG).[6] The diagnostic usefulness of detection of GM antigen in bronchoalveolar lavage (BAL) and in serum is different, but a global experience and consensus on optical density (OD) cutoffs, especially for BAL GM remains lacking. Two cutoffs for BAL GM OD indices (ODIs) have been proposed; ≥0.5 and ≥1.0, but a general consensus on an optimum cutoff value is still unavailable. With this aim, the following study was conducted to compare the performance of BAL versus serum GM testing for the diagnosis of IPA in at-risk patients with hematological malignancies and to define optimum cutoff values for BAL cutoff values in such group of patients.
Methods
This prospective case–control study was conducted in a tertiary care hospital in New Delhi to determine an optimal BAL GM cutoff for IPA in at-risk patients. The period of study was 2 years, The period of study was three years.
Cases were subjects with hematological diagnoses who met established revised definitions for proven or probable IPA established by the EORTC/MSG, 2008, without the use of BAL GM results.
Exclusion criteria were a prior use of antibiotic (piperacillin-tazobactam) and use of antifungals that were active against Aspergillus species before bronchoscopy.
Two control groups were included: patients with hematological diagnoses not meeting definitions for proven or probable IPA and patients with nonhematological diagnoses with no evidence of aspergillosis. The controls were matched in terms of age, gender, and sex.
Following bronchoscopy and sampling of BAL, GM testing was performed using the Platelia Aspergillus seroassay in accordance with the manufacturer's instructions. The BAL sample of both patients and controls was collected by a similar technique, using a fiber-optic bronchoscope and sterile technique. In brief, 10–15 ml of BAL fluid was collected aseptically and sent to the laboratory. A volume of 300 μl of BAL fluid and 100 μl of treatment solution were boiled for 3 min. After centrifugation at 10,000 g for 10 min and successive steps of adding a conjugated and incubation with rat monoclonal antibody, the OD and ODI values were calculated. Statistical parameters such as sensitivity, specificity, negative and positive predictive values, K correlation, likelihood ratios, and diagnostic odds ratios, along with their associated 95% confidence intervals (CIs), were calculated for BAL GM testing (at different OD cutoff values).
The informed written consent of patient enrolled in the study was obtained.
Results
A total of 51 BAL samples from patients suffering from hematological malignancies were analyzed by Platelia EA assay.
Of total 51 cases of IPA as per EORTC/MSG guidelines, the following was the distribution of cases: proven IPA: 2/51 (3.92%), probable IPA: 38/51 (74.5%), and possible IPA: 11/51 (21.5%).
The mean age of the patients was 38.6 years (range 15–62 years) and the male-to-female ratio was 1.21. Most common hematological malignancy was acute myeloid leukemia followed by acute lymphoid leukemia.
The mean age of the controls was 34.9 years (range 16–49 years) and the male-to-female ratio was 1.22.
All patients had positive radiological findings suggestive of invasive aspergillosis; ground glass opacities (32%), bilateral ground glass opacities (28%), halo sign (20%), diffuse reticular alveolar opacities (8%), nodular lesions (8%), and pleural effusion (4%).
Mean BAL-GM ODI in patients was 1.27 (range 0.4–3.67) and mean serum-GM ODI was 0.7 (range 0.2–2.87).
The mean BAL- GM ODI with Aspergillus flavus, Aspergillus Fumigatus, and Aspergillus niger were 1.43, 1.47, and 2.81 respectively. The mean serum-GM ODI with A. flavus, Aspergillus Fumigatus, and A. niger was 0.87, 0.91, and 1.74 respectively.
Mean BAL-GM ODI in controls was 0.262 (range 0.09–0.4) and mean serum-GM ODI was 0.178 (range 0.1–0.4). All patients with BAL-GM ODI >0.5 had a positive culture result (potassium hydroxide positive and growth of Aspergillus species).
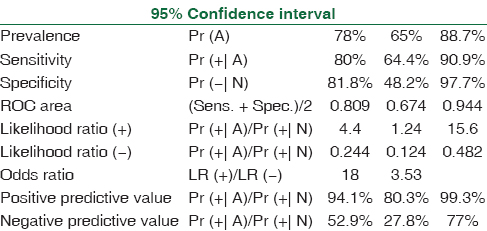
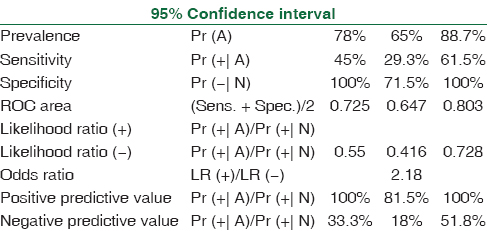
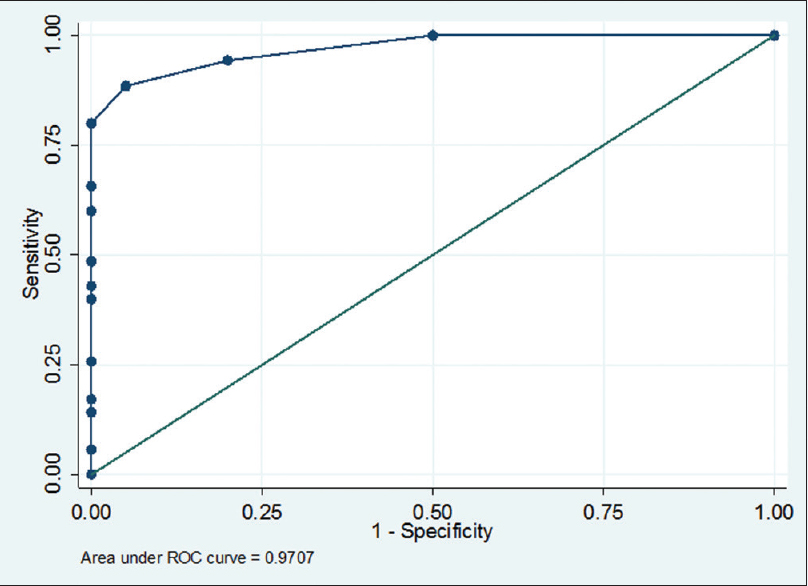
The spectrum of Aspergillus infections was A. flavus (53%) > Aspergillus fumigatus (41%) > A. niger (4%). Receiver operating characteristic curve of BAL ODI and serum ODI predicting invasive aspergillosis (95% CI of area under the curve: 0.95–1.00) [Tables 1–4, Figures 1 and 2] indicates that increase of BAL GM ODI cutoff to >1.00 will add to the identification of Aspergillus species as a causative agent of IPA in hematology patients.

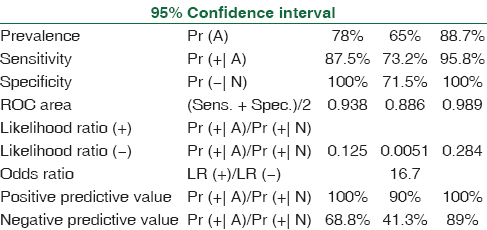
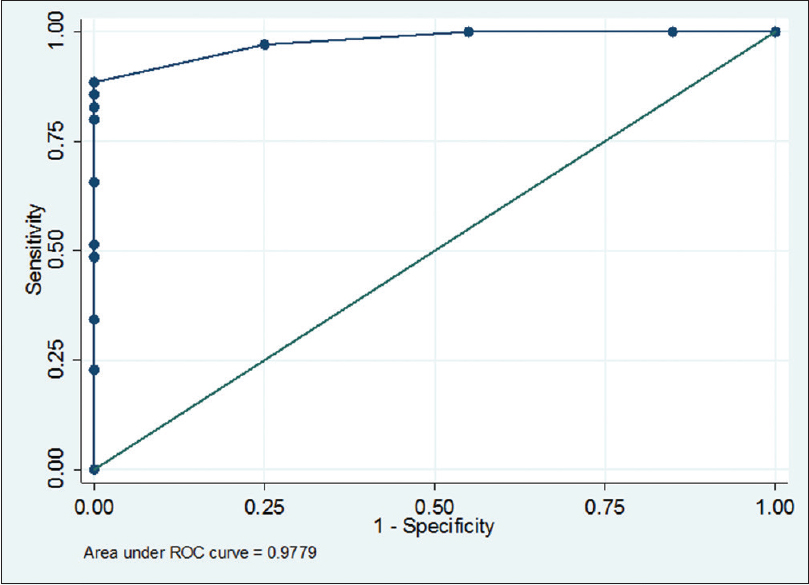
- Receiver operating characteristic curve of bronchoalveolar lavage-optical density index predicting invasive aspergillosis (95% confidence interval of area under the curve: 0.95–1.00) cutoff 0.7 (sensitivity 88.5% and specificity 100%)
- Receiver operating characteristic curve of serum optical density index predicting invasive aspergillosis (95% confidence interval of area under the curve: 0.94–1.00) cutoff 0.4 (sensitivity 88.6% and specificity 95%)
Discussion
The diagnosis of invasive aspergillosis is done by various parameters using of EORTC guidelines (2008), wherein the disease is categorized as proven/probable/possible. In patients suffering with hematological malignancies, lung biopsy may be contraindicated due to the risk of bleeding and associated thrombocytopenia. Relying on culture to diagnose a fungal infection in such category of patients translates into a poor outcome due to their limited sensitivity and false positivity due to colonization of airways by conidia. Radiological signs may be nonspecific to confirm an infection. Current evidence suggests that antifungal therapy should be initiated as early as possible as the disease is diagnosed to improve outcome. Nonculture-based methods including detection of GM in body fluids (e.g., BAL, peritoneal fluid, cerebrospinal fluid, etc.) other than serum and plasma by a commercially based enzyme immunoassay, and β-glucan serum, and fungal DNA in body fluids by PCR for diagnosis of IPA have shown a tremendously overwhelmingly response.[7891011]
The presence of GM in BAL may be affected by various factors. False positivity may be attributed to invasive fungal infections caused by Penicillium marneffei, Cryptococcus neoformans, Geotrichum capsulatum, Histoplasma capsulatum, or Lichtheimia ramosa; due to drugs such as piperacillin-tazobactam; in patients with intestinal mucositis due to chemotherapy and irradiation; or in patients receiving Plasma-Lyte for intravenous hydration, and false negative in patients with chronic granulomatous disease and Job's syndrome. Patients who are at high risk, such as stem cell transplant recipients or with prolonged neutropenia, may be screened for the development of invasive Aspergillus infection by monitoring serum GM levels weekly.[12] One study of serum GM index (GMI) found that clinical outcome may be predicted by elucidating the trends of GM indices during the first 2 weeks of antifungal therapy. A reduction in GMI between baseline and week 1 was associated with a good clinical response.[13] The presence of an elevated GM level in BAL fluid may also be helpful in the diagnosis of pulmonary aspergillosis in patients in whom compatible radiographic changes are present, and BAL testing is performed in the suspicious area.[14] A meta-analytical study and systematic review show that the measurement of BAL-GM levels may help in early diagnosis of invasive aspergillosis.[15] Our results also support BAL GM testing as a reasonably safe test with higher sensitivity compared to serum GM testing in at-risk patients with hematological diseases.
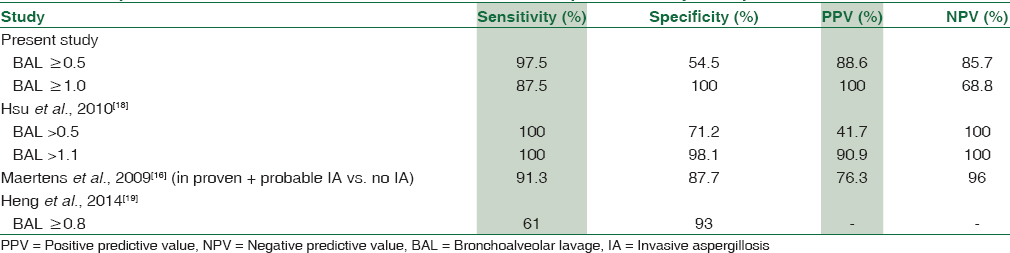
Our study also demonstrates that a higher BAL OD cutoff is necessary to avoid overdiagnosis of IPA in hematological malignancies. Few studies provide an agreement to fact that raising the BAL cutoff to > 1 will increase the sensitivity of detection of IPA (91%) and also rules out IPA with high negative predictive value (96%).[16] The comparison of our study with other studies also provided in Table 5. Studies have also shown as bronchial wash may yield a higher value of GM as compared to BAL.[17]
The present study shows that an increased sensitivity of detection of IPA by assay of GM in BAL as compared to serum. This may be explained by two factors of dynamics of fungal infection; first by a high conidial burden in bronchial tree, secreting a higher quantity of GM, and second that during fungal infection, airway invasion precedes the invasion of blood vessels, thereby accounting for higher GM in BAL.
Our study also shows that though increasing BAL GM cutoff to >1 (as compared to >0.5) is associated with a slight decrease in sensitivity (87.5% vs. 97.5%) but a significant rise in specificity (100% vs. 54.5%). This is an agreement with previous studies (Hsu et al. 2000), which also concluded that increasing the detection cutoff will prevent overdiagnosis of IPA.
Studies with higher sample numbers will allow for better discrimination of values for detection. A uniformity of cohort of patients who are naïve to antifungal therapy, in whom bronchoscopy have been performed timely and adequately (with regard to technique and volume of saline used for sampling) will add to the study. Serial follow-up of patients may help in assessing response to antifungal therapy.
Conclusions
Our study concludes taking a higher cutoff OD of GM detection in BAL at >1 will increase the specificity of detection in IPA. Measurement of circulating GM in BAL fluid will expedite the diagnosis of IPA and is likely to assist in the medical and pharmacological management of the patient.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.19
References
- Pathogenesis of Aspergillus fumigatus in invasive aspergillosis. Clin Microbiol Rev. 2009;22:447-65.
- [Google Scholar]
- Invasive aspergillosis: Epidemiology, diagnosis and management in immunocompromised patients. Drugs. 2007;67:1567-601.
- [Google Scholar]
- Clinical risk factors for invasive aspergillosis. Med Mycol. 2011;49(Suppl 1):S7-12.
- [Google Scholar]
- Invasive aspergillosis in patients with acute leukemia: Update on morbidity and mortality – SEIFEM-C report. Clin Infect Dis. 2007;44:1524-5.
- [Google Scholar]
- Use of circulating galactomannan screening for early diagnosis of invasive aspergillosis in allogeneic stem cell transplant recipients. J Infect Dis. 2002;186:1297-306.
- [Google Scholar]
- Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive fungal infections Cooperative Group and the National Institute of Allergy and infectious diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813-21.
- [Google Scholar]
- Comparison of serum galactomannan and 1,3-beta-D-glucan determination for early detection of invasive pulmonary aspergillosis in critically ill patients with hematological malignancies and septic shock. Mycopathologia. 2016;181:505-11.
- [Google Scholar]
- Correction: Galactomannan testing and the incidence of invasive pulmonary aspergillosis: A 10-year nationwide population-based study in Taiwan. PLoS One. 2016;11:e0156566.
- [Google Scholar]
- Aspergillus PCR in serum for the diagnosis, follow-up and prognosis of invasive aspergillosis in neutropenic and nonneutropenic patients. Clin Microbiol Infect. 2016;22:562.e1-8.
- [Google Scholar]
- Screening for circulating galactomannan as a noninvasive diagnostic tool for invasive aspergillosis in prolonged neutropenic patients and stem cell transplantation recipients: A prospective validation. Blood. 2001;97:1604-10.
- [Google Scholar]
- Diagnosis of invasive aspergillosis using a galactomannan assay: A meta-analysis. Clin Infect Dis. 2006;42:1417-27.
- [Google Scholar]
- Early serum galactomannan trend as a predictor of outcome of invasive aspergillosis. J Clin Microbiol. 2012;50:2330-6.
- [Google Scholar]
- Galactomannan detection in computerized tomography-based broncho-alveolar lavage fluid and serum in haematological patients at risk for invasive pulmonary aspergillosis. Br J Haematol. 2003;121:448-57.
- [Google Scholar]
- Accuracy of BAL galactomannan in diagnosing invasive aspergillosis: A bivariate metaanalysis and systematic review. Chest. 2010;138:817-24.
- [Google Scholar]
- Bronchoalveolar lavage fluid galactomannan for the diagnosis of invasive pulmonary aspergillosis in patients with hematologic diseases. Clin Infect Dis. 2009;49:1688-93.
- [Google Scholar]
- Galactomannan antigen detection using bronchial wash and bronchoalveolar lavage in patients with hematologic malignancies. Ann Clin Microbiol Antimicrob. 2015;14:50.
- [Google Scholar]
- Galactomannan testing of bronchoalveolar lavage fluid is useful for diagnosis of invasive pulmonary aspergillosis in hematology patients. BMC Infect Dis. 2010;10:44.
- [Google Scholar]
- Clinical utility of Aspergillus galactomannan and PCR in bronchoalveolar lavage fluid for the diagnosis of invasive pulmonary aspergillosis in patients with haematological malignancies. Diagn Microbiol Infect Dis. 2014;79:322-7.
- [Google Scholar]





