Translate this page into:
Comparison of arterial and capillary blood glucose measured using a glucometer and blood gas analyzer, and its association with acuity of illness: A longitudinal cohort study
*Corresponding author: Lakshmi Ramamoorthy, Department of Medical Surgical Nursing, College of Nursing, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry, India. laxmi_ramamoorthy@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Deka N, Ramamoorthy L, Ramsankar P, Harichandrakumar KT, Lalthanthuami HT. Comparison of arterial and capillary blood glucose measured using a glucometer and blood gas analyzer, and its association with acuity of illness: A longitudinal cohort study. J Lab Physicians. 2024;16:475-82. doi: 10.25259/JLP_83_2024
Abstract
Objectives:
Dysglycemia is one of the major complications in the critical care unit. Many factors interfere with the accuracy of blood glucose measurements among critically ill patients; therefore, results must be interpreted with caution. This longitudinal cohort study assessed the level of agreement between blood glucose values obtained from capillary and arterial blood using a glucometer over time and its association with the Acute Physiology and Chronic Health Evaluation-II score.
Materials and Methods:
Eighty-one participants were selected by convenience sampling method. Arterial and capillary blood blood glucose levels were measured at three points of time (i.e. on intensive care unit admission, at 24 hours, and 36 hours after cardiac surgery) using a Control-D glucometer and blood gas analyzer.
Statistical analysis:
Inferential analyses like two-way repeated measures analysis of variance, Wilcoxon’s signed rank test, intraclass correlation coefficient, and receiver operating characteristics analysis were used.
Results:
The study results revealed that there was a good agreement between the arterial and capillary blood sugar measured using a glucometer (intraclass correlation coefficient ranges from 0.95 to 0.98). The difference between the blood glucose levels measured using different methods was comparable (P > 0.05) during the admission at the range of hyperglycemia. However, at 24 and 36 h after cardiac surgery, the capillary blood significantly (P < 0.05) overestimated blood glucose level (by18–22 mg/dL and 30 mg/dL) as compared to arterial blood glucose measured using a glucometer and blood gas analyzer.
Conclusions:
Arterial blood in a glucometer can be used for measuring blood glucose levels in a critical care setting whenever an arterial access is available, or when a blood gas analyzer is not accessible.
Keywords
Blood glucose
Arterial blood glucose
Capillary blood glucose
Critical care
Glucometer
Blood gas analysis
Longitudinal studies
INTRODUCTION
The advancement of technology and emerging trends in critical care settings has enormously improved the approach toward the care of critically ill patients in terms of efficiency and effectiveness of care and management provided to the patients. Patients who are critically ill are predisposed to a wide range of complications due to the seriousness of their underlying illness and the requirement for intensive care therapies. Among those, one of the major complications among patients admitted to the critical care unit is dysglycemia, which includes stress hyperglycemia, hypoglycemia (most frequent), and glycemic variability.[1]
Since long back, it has been well evidenced that blood glucose levels correlate with the prognostic outcome of critically ill patients[2] and are more likely to experience severe stress reactions that lead to stress-induced hyperglycemia documented in 40–60% of general intensive care unit (ICU) patients and 60–80% of patients undergoing cardiac surgery, respectively.[3] Although hyperglycemia can be managed with intensive insulin therapy (IIT), simultaneously, there is an unanticipated risk of hypoglycemia as well among critically ill patients.[4] They are at an independent risk of increased mortality due to hypoglycemia, and the risk rises over time and with the frequency of hypoglycemic episodes, thus implying a need for close and accurate measurement of blood glucose levels, especially for those admitted in the ICUs.
The sampling priority in critically ill patients for glucose monitoring is arterial > venous > capillary.[5] A prospective observational study results found that the accuracy of capillary blood glucose values was only 26.3% as compared to that of arterial blood glucose values measured with a glucose meter or a blood gas analyzer, which was 55.6% and 64.9%, respectively.[6] In a systematic review, the analysis of 21 studies found that blood glucose can be measured more accurately using arterial blood in a blood gas analyzer or a glucose meter than capillary blood, and the accelerated errors were found to be associated with hemodynamic instability, use of vasoconstricting agents, insulin infusion, etc.
Critically ill patients are at higher risk of developing dysglycemia, for which accurate measures to be taken to monitor blood glucose levels. Although various studies have been conducted, uniformity in blood glucose levels in critically ill patients measured with various methods is not well established. Therefore, the present study aims to bring more clarity regarding a device used as a point of care while it is used for blood glucose monitoring, when it is necessarily important to use arterial blood, and in what circumstances it can be replaceable with capillary sample.
MATERIALS AND METHODS
A longitudinal cohort design was adopted for the study. Data were collected from the cardiothoracic and vascular surgery (CTVS) ICU of a tertiary care hospital of the public health sector from South India for a period of 7 months (September 2022–April 2023).
Sampling
Participants were selected using a convenience sampling technique. Anticipating an intra-class correlation coefficient (ICC) in the level of blood glucose obtained from arterial and capillary blood samples as 0.75 at a 5% level of significance and 80% power with a null value of 0.60. The sample size was calculated using the method for agreement on quantitative outcomes (intra-class correlation). A total of 81 participants were enrolled. Adult patients on admission to CTVS ICU after cardiac surgery who were ≥18 years old and expected to have an arterial line at least for the next 36 hours of cardiac surgery were enrolled in the study.
Ethical considerations
Permission was obtained for the present study from the Institute Nursing Research Monitoring Committee (JIP/CON/NRMC/M.Sc./2021/MSN/9) and the Institute Ethics Committee for Human Studies (JIP/CON/IEC/M.Sc./2021/ MSN/8). The procedures followed were in accordance with the ethical standards of the institution as well as the Declaration of Helsinki revised in 2013. After a brief explanation to the legally authorized representative (LAR) of each enrolled patient regarding the study, informed consent was obtained from LAR since patients cannot give consent due to critical condition and inability/unconsciousness. Patient data were stored confidentially. Confidentiality, the anonymity of the subjects, and the right to withdraw from the study were explained to LAR before data collection.
Data collection
Initial data for every participant were collected, which included demographic data, admission diagnosis, comorbidities, date of intubation, and type of arterial access.
Data collected on ICU admission (0 h), 24 h, and 36 h of post-cardiac surgery were – temperature, pulse, respiration, blood pressure, oxygen saturation level, arterial blood – potential hydrogen, partial pressure of carbon dioxide, partial pressure of oxygen, bicarbonate − and lactate level, capillary refill time (CRT), vasoactive drugs and insulin infusion (if any), and blood glucose values from capillary and arterial blood and Acute Physiology and Chronic Health Evaluation (APACHE) II score was calculated using “MDCALC” mobile application.
For arterial blood
Before collecting blood samples from the arterial line, the hub was scrubbed for 15 s with a 70% alcohol solution and kept for 30 s to dry.[7] First, 5 mL of blood from the arterial line was collected in a sterile 5 mL syringe, followed by 0.5 mL of blood in a heparinized syringe, and the blood obtained in the 5 mL sterile syringe was re-infused (as per CTVS ICU protocol), the arterial line was flushed with heparinized saline (1:1) after obtaining the sample. Blood glucose measurement was directly performed with a glucometer (Control-D) and blood gas analyzer (Rapid point 500 e).
For capillary blood
The palm-up surface of the middle and ring fingers was cleansed with 70% Isopropyl alcohol and kept for air drying. A finger prick was done on the side of the fingertip with the help of a 2.2 mm lancet device. [8] The first drop of blood was wiped out, and the second drop was directly collected in a glucose strip, and blood sugar values were measured in a glucometer. Firm pressure with clean gauze was applied until hemostasis was obtained.
Duration of measurements
Blood samples were collected at three points in time: on ICU admission (at 0 h), 24 h, and 36 h after cardiac surgery. At each point, it took approximately 5 minutes to collect blood samples and measure blood glucose values from the abovementioned sites.
The blood sugar values obtained from the arterial line and capillary blood were measured with a glucometer and arterial blood in a blood gas analyzer at all three-time points: 0 h, 24 h, and 36 h.
Data analysis
All the statistical analyses were done using the Statistical Package for the Social Sciences version 19 on an intention-to-treat basis. The distribution of categorical data was expressed as frequencies and percentages. Continuous variables were expressed as mean with standard deviation or median with interquartile range. The agreement in the blood glucose level obtained from arterial and capillary blood samples was assessed using the ICC. The comparison of median blood glucose levels obtained using different samples and methods at each time point was carried out using one-way repeated measures analysis of variance. The level of agreement between blood glucose values from different blood samples across APACHE-II scores was also assessed using the ICC method. Comparison of the longitudinal changes in blood glucose values between various samples and methods was performed using a two-way repeated measures analysis of variance.
Blood glucose values >140 mg/dL were classified as hyperglycemic[7] and then sensitivities and specificities for the two blood glucose measurements using a glucometer were obtained, considering the arterial blood glucose measured using a blood gas analyzer as the reference method. Then, a receiver operating characteristics (ROC) analysis was performed, and diagnostic accuracies of arterial blood glucose measured with a glucometer and capillary blood glucose measured using a glucometer were assessed by the area under the ROC curve (AUC). Wilcoxon’s signed-rank test was used to compare the absolute difference in blood glucose levels obtained from different samples and methods between different clinical characteristics. All statistical analyses were carried out at a 5% level of significance.
RESULTS
The study gathered data from 81 participants from CTVS ICUs. The mean age was 46.56 ± 11.3 years. In terms of gender, a male preponderance (58%) was found, and nearly half of the patients had undergone valve replacement surgery (48.1%).
The agreement in the blood glucose levels assessed over time using capillary and arterial blood in the glucometer and arterial blood in the blood gas analyzer was found to have a good agreement at the ICC range of 0.95–0.98 [Figure 1]. The agreement of the blood glucose values measured using different methods of measurement among different categories of APACHE-II score had shown a good agreement with the ICC range of 0.92–0.99 [Table 1].
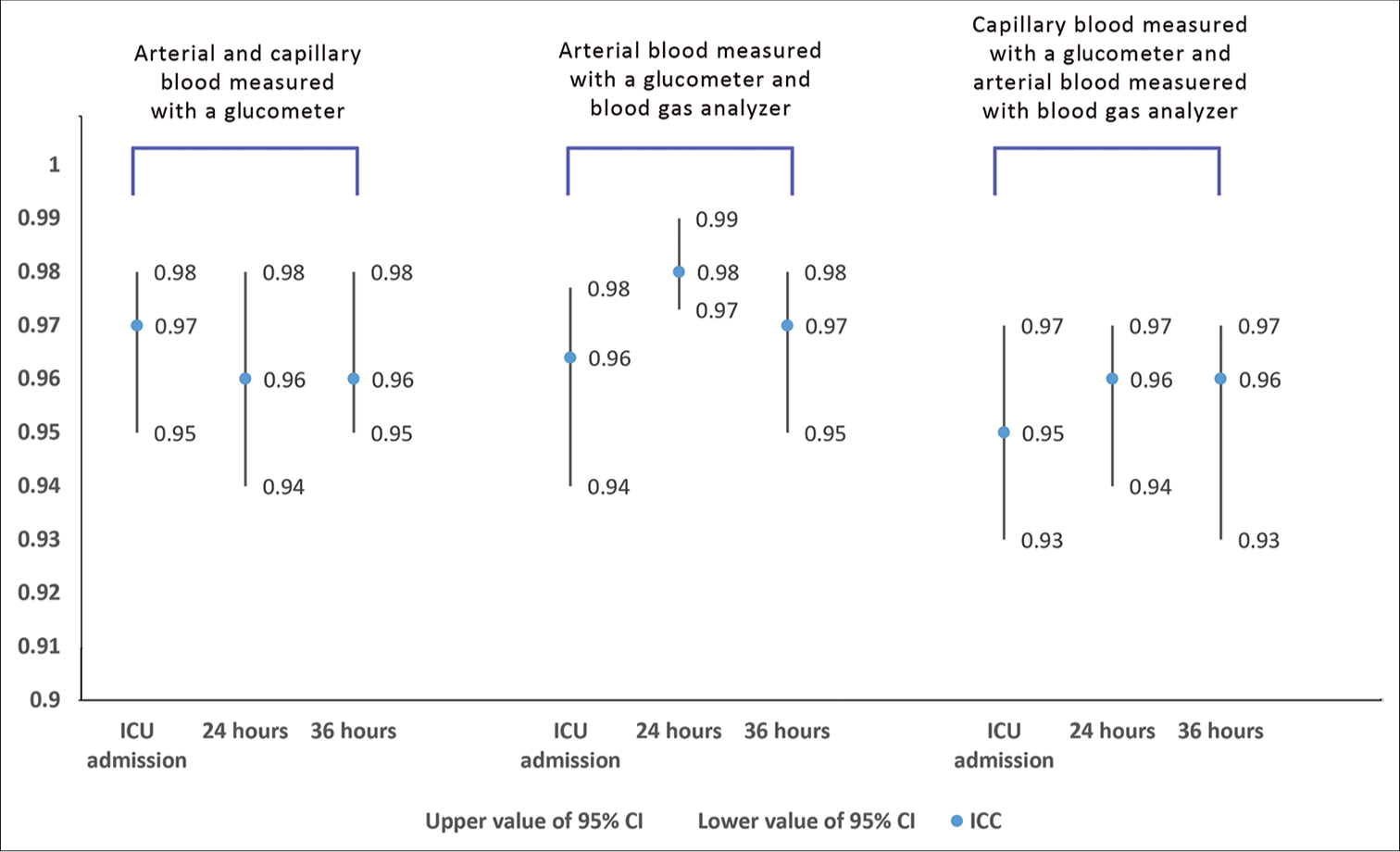
- Agreement in the blood glucose levels assessed over time using capillary and arterial blood in the glucometer and arterial blood in the blood gas analyzer. ICU: Intensive care unit; ICC: Intraclass correlation coefficient, CI: Confidence interval.
| Methods of blood glucose measurement | APACHE-II Score | Intraclass correlation (95% CI) | ||
|---|---|---|---|---|
| 0 h | 24 h | 36 h | ||
| Arterial blood in the glucometer and capillary blood in the glucometer | 0–14 | 0.97 (0.911, 0.99) | 0.96 (0.94, 0.98) | 0.94 (0.91, 0.96) |
| 15–29 | 0.96 (0.93, 0.97) | 0.95 (0.86, 0.98) | 0.98 (0.93, 0.99) | |
| Arterial blood in glucometer and blood gas analyzer | 0–14 | 0.97 (0.92, 0.99) | 0.98 (0.96, 0.98) | 0.96 (0.93, 0.97) |
| 15–29 | 0.96 (0.93, 0.97) | 0.99 (0.97, 0.99) | 0.98 (0.93, 0.99) | |
| Capillary blood in glucometer and arterial blood in blood gas analyzer | 0–14 | 0.96 (0.907, 0.989) | 0.96 (0.95, 0.981) | 0.95 (0.93, 0.97) |
| 15–29 | 0.94 (0.91,0.97) | 0.92 (0.76, 0.97) | 0.95 (0.84, 0.98) | |
APACHE: Acute physiology and chronic health evaluation, CI: Confidence interval
The blood glucose level showed no significant difference when compared among all three methods at the baseline during the state of hyperglycemia and high APACHE-II score. The capillary blood glucose assessed using a glucometer significantly overestimates blood glucose levels compared to arterial blood glucose measured using a glucometer and blood gas analyzer, especially at 24 h and 36 h after cardiac surgery [Figure 2].
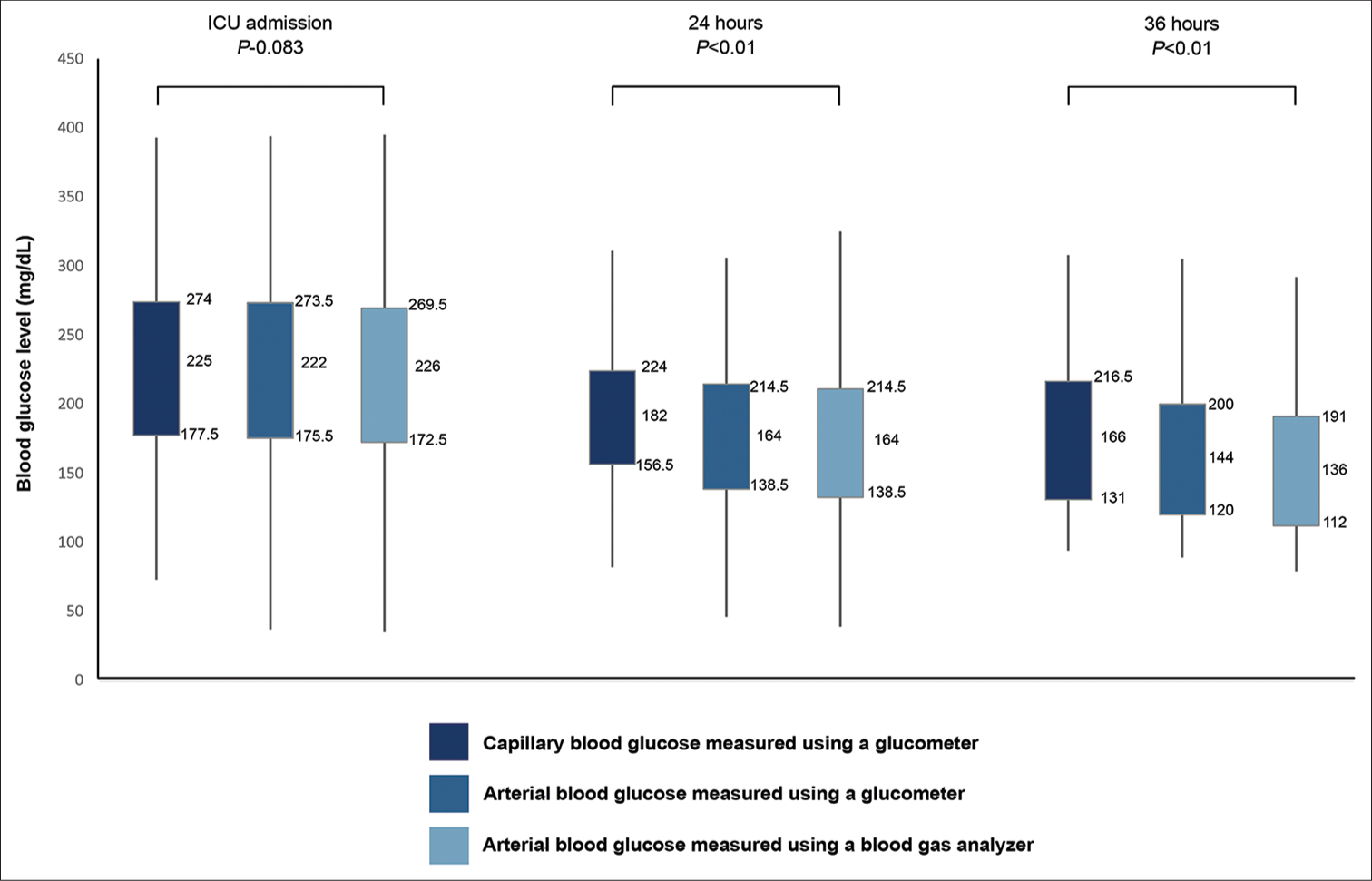
- Relationship between acute physiology and chronic health evaluation score and different methods of blood glucose measurement. ICU: Intensive care unit.
Regarding ROC curve analysis, the discriminatory power of both methods to detect hyperglycemia was good. The arterial blood glucose measured using glucometer showed a higher performance in detecting hyperglycemia (AUC – 96.9%; P = 0.001) when compared to capillary blood glucose measured using glucometer (AUC – 96.4%; P = 0.001) at the time of ICU admission. After 24 h, arterial blood glucose (AUC – 98.1%; P = 0.001) remains more favorable than capillary blood glucose measurement using a glucometer (AUC – 95.6%; P = 0.001). After 36 h of ICU admission, the capillary blood glucose method (AUC – 99.4%; P = 0.001) conversely exhibited a better effect in detecting hyperglycemia when compared to arterial blood glucose measured using a glucometer (AUC – 94.5%; P = 0.001) [Figure 3].
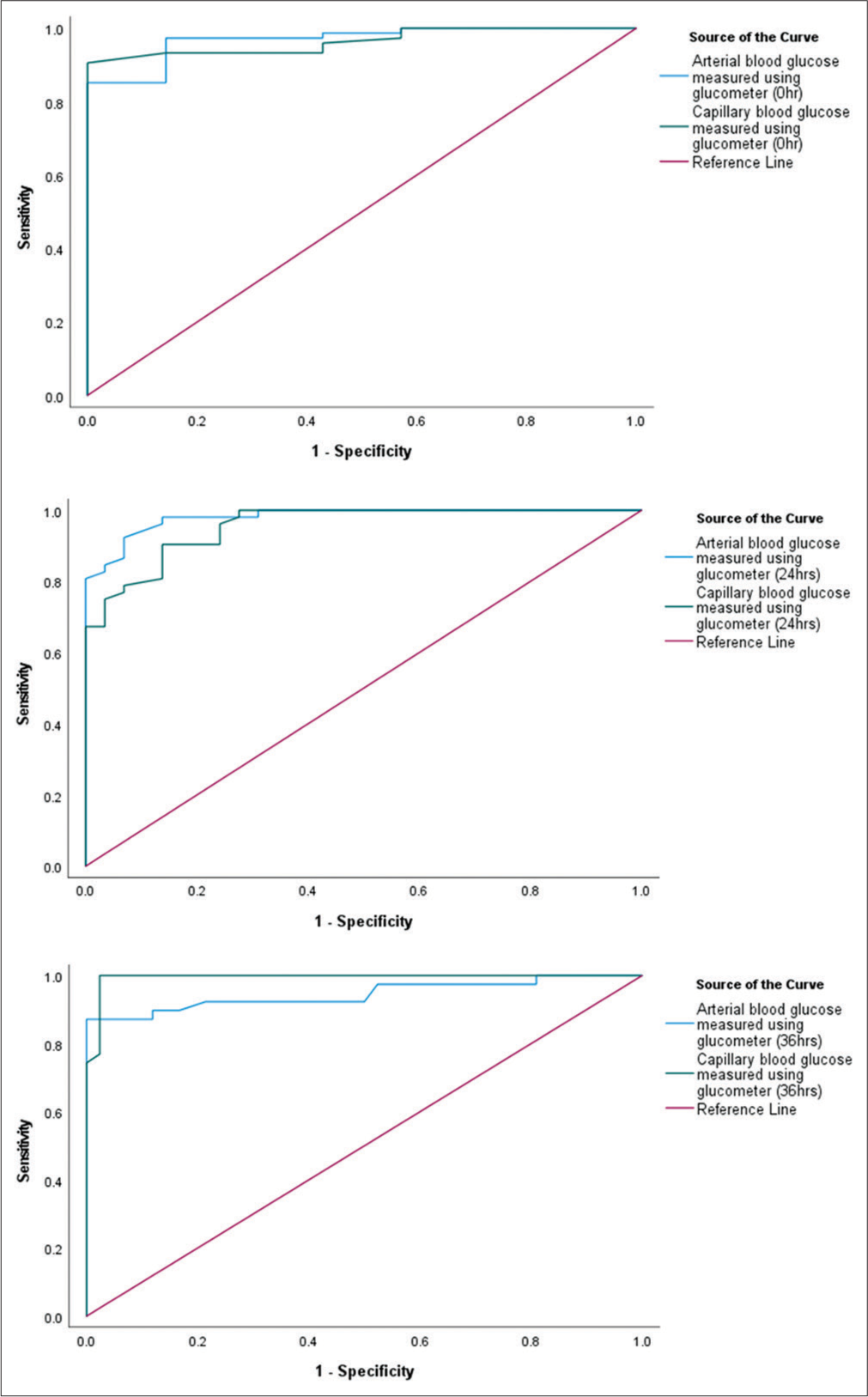
- Receiver operating characteristics curve on different methods of blood glucose measurement.
Arterial blood glucose measured using a glucometer and blood gas analyzer provides more consistent blood glucose values over time than capillary blood glucose assessed using a glucometer [Table 2].
| Methods of blood glucose measurements | On ICU admission | 24 h | 36 h | P-value* |
|---|---|---|---|---|
| Arterial and capillary blood in glucometer | 222 (175.5, 273.5) | 164 (138.5, 214.5) | 144 (120, 200) | <0.01 |
| 225 (177.5, 274) | 182 (156.5, 224) | 166 (131, 216.5) | ||
| Capillary blood in glucometer and arterial blood in blood gas analyzer | 225 (177.5, 274) | 182 (156.5, 224) | 166 (131, 216.5) | <0.01 |
| 226 (172.5, 269.5) | 152 (132.5, 211) | 136 (112, 191) | ||
| Arterial blood in glucometer and arterial blood in blood gas analyzer | 222 (175.5, 273.5) | 164 (138.5, 214.5) | 144 (120, 200) | 0.150 |
| 226 (172.5, 269.5) | 152 (132.5, 211) | 136 (112, 191) |
Acidosis at 24 h, inotropes at 24 h, and lactacidemia at 36 h are potential confounding factors that may contribute to the absolute difference in the measurement of blood glucose levels using various methods [Table 3].
| Clinical parameters | Absolute difference in blood glucose level between different samples and methods (mg/dL) | |||||
|---|---|---|---|---|---|---|
| Arterial and capillary blood measured using a glucometer | Arterial blood measured using a blood gas analyzer and capillary blood using a glucometer | Arterial BG measured using a glucometer and blood gas analyzer | ||||
| Median (IQR) | P-value | Median (IQR) | P-value | Median (IQR) | P-value | |
| Hypotension at 0 h | ||||||
| Yes | 16 (7,33) | 0.659 | 22 (13, 43) | 0.176 | 20 (26, 3) | 0.912 |
| No | 15 (8, 25.25) | 16 (6.75, 32) | 12 (6, 27) | |||
| Acidosis at 0 h | ||||||
| No | 13 (7, 18) | 0.106 | 16 (6, 22) | 0.413 | 10 (5, 27) | 0.869 |
| Yes | 16.5 (12.25, 28.75) | 16.5 (8, 38.75) | 12 (6, 25.75) | |||
| Alkalosis | 13 (4.5, 22.25) | 20 (6.5, 38) | 17.5 (5.5, 26.75) | |||
| Acidosis at 24 h | ||||||
| No | 16 (9, 32) | 0.031* | 24 (15, 32) | 0.163 | 10 (3, 22) | 0.715 |
| Yes | 12.5 (3, 31.75) | 20 (11.75, 23.75) | 10.5 (6.25, 45.5) | |||
| Alkalosis | 12 (8.5, 29.5) | 23.5 (18.75, 41) | 13.5 (7.5, 19.25) | |||
| Acidosis at 36 h | ||||||
| No | 11 (6, 21.5) | 0.621 | 22.5 (13.75, 31.25) | 0.338 | 11.5 (6.5, 18.25) | 0.477 |
| Yes | 19.5 (9.5, 36.25) | 35 (10.75, 71.5) | 14 (5.75, 42) | |||
| Alkalosis | 14 (10, 29.5) | 25 (13.5, 35) | 15 (8, 24) | |||
| Lactacidemia at 24 h | ||||||
| Yes | 17.5 (8.25, 33) | 0.659 | 25 (15, 38.75) | 0.541 | 10.5 (5.25, 24) | 0.722 |
| No | 14 (9, 31) | 22 (17, 29.5) | 13 (4.5, 19.5) | |||
| Lactacidemia at 36 h | ||||||
| Yes | 17 (11, 32) | 0.315 | 35 (25, 52) | 0.004* | 18 (14, 30) | 0.016* |
| No | 11 (7, 25) | 21 (11, 33) | 11.5 (6, 19) | |||
| Inotropes at 24 h | ||||||
| Yes | 16.5 (9, 34) | 0.124 | 25 (17.75, 37.25) | 0.017* | 10.5 (5.75, 22.25) | 0.951 |
| No | 11 (8, 21) | 15 (9, 20) | 13 (3, 20) | |||
| Inotropes at 36 h | ||||||
| Yes | 12 (10, 25) | 0.571 | 27 (11, 41.5) | 0.250 | 14 (7.5, 21.5) | 0.634 |
| No | 11 (6, 28.5) | 19.5 (14, 30) | 12 (7, 20.5) | |||
BG: Blood glucose, IQR: Interquartile range, Wilcoxon signed-rank test. Bold value: *P<0.05
DISCUSSION
The “Surviving Sepsis Campaign” 2016 in the international conference recommends the use of arterial blood in a glucometer for blood glucose measurement for critically ill patients, though, with a weak recommendation and low-quality evidence, the recommendation was made from the best available practices.[7,9] A recent survey assessed the response from 72 specialists, which shows that 51% of blood glucose measurement is done by point-of-care glucometer devices with unspecified blood sample sites, but the majority of the samples suggested measurement probably with capillary blood samples.[10] To prevent errors in measurement with capillary blood glucose measured using a glucometer, especially in critically ill patients, the practice of using capillary blood samples for glucose measurement must be eliminated.[11,12]
Previous studies have already mentioned blood gas analyzers as the gold standard for blood glucose measurements for critically ill patients. [2,7,9,13] The present study assesses blood glucose levels and their agreement using capillary and arterial blood in a glucometer and arterial blood in a blood gas analyzer. The agreement among the various methods of measurement has shown good agreement (ICC ranges from 0.95 to 0.98). A similar study was conducted, and the results showed a moderate agreement of blood glucose levels among arterial, central venous, and capillary blood glucose levels measured using a glucometer.[7]
A prospective case-control study was performed to compare the arterial and capillary blood glucose monitoring in 100 participants in both normotensive and shock with a vasopressor support group. The study results found more than 20% disagreement with significantly different values between the two methods of measurement.[14] In this present study findings, the result shows that though there is a good agreement between the blood glucose values measured using capillary and arterial blood in a glucometer and arterial blood in a blood gas analyzer, there is a significant difference in the blood glucose values measured using the methods mentioned above. Another study was conducted to compare the differences, correlation, and consistency between blood glucose levels assessed using a glucometer and blood gas analyzer among critically ill patients. The result showed significant differences in both methods but with a good level of agreement.[15]
On the contrary, a similar study reported a moderate agreement of blood glucose levels among critically ill patients with an absolute error difference for disagreement of 34 mg/dL (10%). The result reported an underestimation of blood glucose in capillary blood as compared to arterial and central venous values.[7] The present study findings show that capillary blood glucose in glucometer significantly overestimates as compared to arterial blood glucose irrespective of the methods of measurement used, especially during the follow-up. The longitudinal changes of the blood glucose values when compared with all three methods of measurement, that is, arterial blood glucose in the glucometer, capillary blood glucose in the glucometer, and arterial blood glucose in the blood gas analyzer, show that the blood glucose levels had no significant difference at the baseline when measured with all the method mentioned above., whereas capillary blood glucose level was significantly higher as compared to the other methods at 24 h and 36 h of cardiac surgery.
Previous studies have established that a blood gas analyzer and glucometer measure arterial blood glucose more accurately than capillary blood glucose, whereas arterial blood glucose in a blood gas analyzer provides a more accurate measurement of arterial blood glucose than a glucometer. At the range/near hypoglycemia, the rate of error for assessing arterial blood glucose is low when assessed with a blood gas analyzer as compared to other methods.[16] The present study results compare the longitudinal changes in blood glucose levels over time using arterial and capillary blood in a glucometer and arterial blood in a blood gas analyzer. It shows that there was no significant difference in the blood glucose levels during the hyperglycemic state at the baseline when it was compared to all three methods. Although the change in the blood glucose level was higher during the normoglycemia/hypoglycemic range, the arterial blood glucose, irrespective of the methods of measurement, provides significantly more consistent blood glucose values over time as compared to capillary blood glucose.
Since long back, it has been well evidenced that blood glucose levels correlate with the prognostic outcome of critically ill patients[2] and are more likely to experience severe stress reactions that lead to stress-induced hyperglycemia documented in 40–60% of general ICU patients and 60–80% of patients undergoing cardiac surgery, respectively.[4,17] The present study results show a significant decrease in blood glucose levels over time, ranging from hyperglycemia at the baseline with a high APACHE-II score to a normoglycemic state during follow-up with a decrease in APACHE-II score. However, the correlation of APACHE-II scores and blood glucose values assessed by various methods using arterial and capillary blood at various points in time shows no significant correlation. Similarly, the agreement between the blood glucose levels assessed using various methods has shown a good agreement with different categories of APACHE-II scores. However, there was both clinically and statistically significant difference among the blood glucose levels assessed using different methods where capillary blood glucose assessed using a glucometer overestimated blood glucose value, especially at 24 and 36 h of cardiac surgery when the mean APACHE score and mean blood glucose values were significantly decreased clinically from the baseline.
The arterial blood glucose measured using glucometer showed a higher performance in detecting hyperglycemia (AUC – 96.9%, 98.1%; P = 0.001, P = 0.001) when compared to capillary blood glucose measured using glucometer (AUC – 96.4%, AUC – 95.6%; P = 0.001, P = 0.001) at the time of ICU admission and 24 h, respectively. After 36 h of ICU admission, the capillary blood glucose method (AUC – 99.4%; P = 0.001) conversely exhibited a better effect in detecting hyperglycemia when compared to arterial blood glucose measured using glucometer (AUC – 94.5%; P = 0.001). The same has been reported in similar studies.[7-9] ROC analysis for the detection of normoglycemia and hypoglycemia is not included. ROC analysis for the detection of normoglycemia and hypoglycemia is not included due to the concern that accurate identification of cases might not be possible as a result of limited number of samples. This imbalance can significantly affect the model performance and the reliability of the ROC curve.
Previous studies have established the effects of vasoconstrictors, hemodynamic status, hematocrit level, lactic acidosis, CRT, insulin infusion, etc., on the level of agreement of blood glucose assessed using arterial and capillary blood.[14,18,19]
In this study, the result shows a significant (P < 0.05) difference between the arterial and capillary blood glucose, especially at 24 h and 36 h after cardiac surgery. The factors that were found to be significant when compared with the absolute difference among the methods of blood glucose measurements are acidosis at 24 h, lactacidemia at 36 h, and inotropes at 24 h.
CONCLUSIONS
The emerging trends and technology in the field of critical care have improved drastically in terms of efficient and effective patient care and management nowadays, but a genuine solution for an accurate method of assessing blood glucose levels in critically ill patients is still in a dilemma. Although some of the hospitals and critical care settings have accepted the use of arterial blood for measuring blood glucose levels in critically ill patients around the world due to its accuracy and minimal chances of error, it is high time to adopt the same in our health settings, too. Therefore, blood glucose measurement, especially for critically ill patients, should use arterial blood in a glucometer or blood gas analyzer for patients at potential risk of developing hypoglycemia.
Ethical approval
The research/study was approved by the Institutional Review Board at JIPMER Nursing Research Monitoring Committee (JIP/CON/NRMC/M.Sc./2021/MSN/9) and the Institute Ethics Committee for Human Studies (JIP/CON/IEC/M.Sc./2021/MSN/8).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Intramural fund received for reagents (INR 40,400/).
References
- Dysglycemia in the critically ill patient: Current evidence and future perspectives. Rev Bras Ter Intensiva. 2017;29:364-72.
- [CrossRef] [Google Scholar]
- Accuracy of blood glucose measurement and blood glucose targets. J Diabetes Sci Technol. 2020;14:553-9.
- [CrossRef] [Google Scholar]
- Perioperative management of hyperglycemia and diabetes in cardiac surgery patients. Endocrinol Metab Clin N Am. 2018;47:203-22.
- [CrossRef] [Google Scholar]
- Expert consensus on the glycemic management of critically ill patients. J Intensive Med. 2022;2:131-45.
- [CrossRef] [Google Scholar]
- Arterial catheter setup for glucose control in critically ill patients: A randomized controlled trial. Am J Crit Care. 2014;23:150-9.
- [CrossRef] [Google Scholar]
- Reliability of point-of-care testing for glucose measurement in critically ill adults. Crit Care Med. 2005;33:2778-85.
- [CrossRef] [Google Scholar]
- Cut-off value of random blood glucose among Asian Indians for preliminary screening of persons with prediabetes and undetected type 2 diabetes defined by the glycosylated haemoglobin criteria. J Diabetes Clin Res. 2019;1:53-8.
- [CrossRef] [Google Scholar]
- WHO guidelines on drawing blood: Best practices in phlebotomy. Available from: https://www.ncbi.nlm.nih.gov/books/NBK138654 [2021 Oct 19]
- [Google Scholar]
- Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45:486.
- [CrossRef] [Google Scholar]
- Opinions and practices of blood glucose control in critically ill patients with pre-existing type 2 diabetes in Australian and New Zealand intensive care units. Aust Crit Care. 2019;32:361-5.
- [CrossRef] [Google Scholar]
- Accuracy of bedside capillary blood glucose measurements in critically ill patients. J Intensive Care. 2007;33:2079-84.
- [CrossRef] [Google Scholar]
- Accuracy of point-of-care blood glucose measurements in critically ill patients in shock. J Diabetes Sci Technol. 2014;8:937-44.
- [CrossRef] [Google Scholar]
- Blood glucose measurement in the intensive care unit: What is the best method? J Diabetes Sci Technol. 2013;7:489-99.
- [CrossRef] [Google Scholar]
- Comparison between arterial and capillary blood glucose monitoring in patients with shock. Eur J Intern Med. 2011;22:241-4.
- [CrossRef] [Google Scholar]
- A comparison of arterial blood glucose and peripheral blood glucose levels in critically ill patients: Measurements using the arterial blood gas analyzer and the rapid glucose meter. Ann Palliat Med. 2021;10:3179-84.
- [CrossRef] [Google Scholar]
- Accuracy of blood-glucose measurements using glucose meters and arterial blood gas analyzers in critically ill adult patients: Systematic review. Crit Care. 2013;17:R48.
- [CrossRef] [Google Scholar]
- Validity of bedside blood glucose measurement in critically ill patients with intensive insulin therapy. Indian J Crit Care Med. 2016;20:653-7.
- [CrossRef] [Google Scholar]
- Glycemic control in critically ill: A moving target. Indian J Crit Care Med. 2014;18:229-33.
- [CrossRef] [Google Scholar]
- Validity of bedside blood glucose measurement in critically ill patients with intensive insulin therapy. Indian J Crit Care Med. 2016;20:653-7.
- [CrossRef] [Google Scholar]






