Translate this page into:
Comparison of four phenotypic methods for detection of metallo-β-lactamase-producing Gram-negative bacteria in rural teaching hospital
Address for correspondence: Dr. Sweta Sunil Oza, Department of Microbiology, C.U. Shah Medical College and Hospital, Surendranagar, Gujarat - 363 001, India. E-mail: swetaprajapati83@yahoo.in
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
CONTEXT:
Metallo-β-lactamase (MBL)-producing bacteria lead to resistance to carbapenem an antibiotic that used as the last resort for treatment of multidrug-resistant bacteria, extended spectrum beta-lactamases, and AmpC β-lactamase-producing Gram-negative bacteria (GNB). The emergence of MBL-producing GNB is challenge to microbiology laboratories because there are no standardized guidelines available to detect them. The aim of this study was to compare four phenotypic methods to detect MBL production in GNB and to determine antibiotic sensitivity of MBL-producing isolates.
MATERIALS AND METHODS:
A total of 107 clinical isolates of GNB were tested for MBL production. Imipenem (IPM)-resistant GNB were taken as positive for MBL screening. MBL detection was done using ethylene diamine tetra acetic acid (EDTA) as MBL inhibitor. Four phenotypic methods were evaluated: (1) Combined disk synergy test (CDST) with 0.5M EDTA (CDST-0.5 M EDTA), (2) CDST with 0.1 M EDTA (CDST-0.1 M EDTA), (3) double-disk synergy test (DDST) with 0.5M EDTA (DDST-0.5 M EDTA), and (4) DDST with 0.1 M EDTA (DDST-0.1 M EDTA).
RESULTS:
Out of 107 GNB, 30 were resistant to IPM considered as screening positive. Out of 30, 21 (70%) isolates were MBL positive by CDST-0.1 M EDTA, 19 (63.33%) by CDST-0.5M EDTA, 17 (56.67%) by DDST-0.1 M EDTA, and 16 (53.33%) by DDST-0.5M EDTA. All MBL-producing Gram-negative Bacilli were resistant to ampicillin/sulbactam. Polymyxin B was found to be the most sensitive drug.
CONCLUSION:
CDST-0.1 M EDTA is the most sensitive method MBL detection. The detection of MBL-producing GNB is very important to control spread of the resistance.
Keywords
Carbapenem
combined disk test
double-disk test
ethylene diamine tetra acetic acid
Gram-negative bacteria
imipenem
metallo-β-lactamase
Introduction
The introduction of carbapenem for treatment of β-lactam-resistant bacteria is a great achievement in clinical practice.[1] Carbapenems have been used as the last resort of antimicrobials in the treatment of serious infections caused by Gram-negative bacteria (GNB). However, the clinical use of carbapenem is in danger with the emergence of carbapenemases, particularly metallo-β-lactamase (MBL). MBL has the ability to hydrolyze a wide variety of β-lactam agents, such as penicillins, cephalosporins, and carbapenems. MBLs are inhibited by metal chelators, such as an ethylene diamine tetra acetic acid (EDTA) and thiol-based compounds.[2]
MBL-encoding genes have been reported worldwide in GNB, such as Pseudomonas spp., Acinetobacter spp., and members of the Enterobacteriaceae family. MBLs spread easily via plasmids and cause nosocomial infections and outbreaks. Such infections mainly concern patients admitted to the intensive care units with several comorbidities.[3] Therefore, early detection and identification of MBL-producing organisms are of crucial importance for the prevention of nosocomial infection through appropriate treatment.[4]
Detection of genes coding for MBL by polymerase chain reaction (PCR) usually gives reliable and satisfactory results; however, because of the cost the method, it is of limited practical use for routine diagnostic microbiology laboratories. Thus, a simple and inexpensive testing method for detection of MBL producers is necessary.[5] The present study was undertaken to detect MBL in GNB using different phenotypic methods.
Materials and Methods
The present study was done in the Department of Microbiology, Teaching Hospital, from August 2012 to September 2012. A total of 107 GNB isolated from various clinical samples including Escherichia coli, Pseudomonas spp., Klebsiella spp., Acinetobacter spp., and Proteus mirabilis were tested for MBL production. All isolates were subjected to antibiotic susceptibility testing as per the Clinical and Laboratory Standards Institute guidelines.[6]
Imipenem (IPM)-resistant clinical isolates were taken as positive for MBL screening. Isolates which gave MBL screening test positive were subjected to confirmation by four phenotypic methods using EDTA as MBL inhibitor.
A 0.5 M EDTA solution was prepared by dissolving 18.61 g of EDTA in 100 ml of distilled water[137] and 0.1 M EDTA by dissolving 3.72 g of EDTA in 100 ml of distilled water.[8]
Phenotypic methods used for confirmation of MBL production.
Combined disk synergy test with 0.5 M ethylene diamine tetra acetic acid
Two IPM (10 µg) disks were placed 30 mm apart from center to center on the surface of an agar plate, and 10 µl 0.5 M EDTA solution was added to one of them to obtain the desired concentration of 750 µg.[137]
Combined disk synergy test with 0.1 M ethylene diamine tetra acetic acid
Two IPM (10 µg) disks were placed 30 mm apart from center to center on the surface of an agar plate, and 10 µl 0.1 M EDTA solution was added to one of them to obtain the desired concentration of 292 µg.[8]
In combined disk synergy test (CDST), if zone of inhibition of IPM-EDTA disk was ≥7 mm more than that of IPM disk alone, it was considered as MBL positive.[8]
Double-disk synergy test with 0.5 M ethylene diamine tetra acetic acid
An IPM (10 µg) disk was placed 20 mm apart from a blank disk containing 10 µl of 0.5 M EDTA (750 µg).[38]
Double-disk synergy test with 0.1 M ethylene diamine tetra acetic acid
An IPM (10 µg) disk was placed 20 mm apart from a blank disk containing 10 μl of 0.1 M EDTA (292 µg).[8]
In double-disk synergy test (DDST), enhancement of zone of inhibition between IPM and EDTA disk was considered as MBL positive.[137]
Results
Out of 107 clinical isolates of GNB, 30 isolates were resistant to IPM which were considered as MBL screening positive.
All 30 IPM-resistant isolates were further processed for confirmation by different methods for phenotypic detection of MBL producers. In the present study, out of 107 GNB, 21 (19.62%) isolates were positive for MBL production.
Out of 30 isolates, 21 (70%) isolates were MBL positive by CDST-0.1 M EDTA method, 19 (63.33%) by CDST-0.5 M EDTA method, 17 (56.67%) by DDST-0.1 M EDTA method, and 16 (53.33%) by DDST-0.5 M EDTA method [Figure 1].
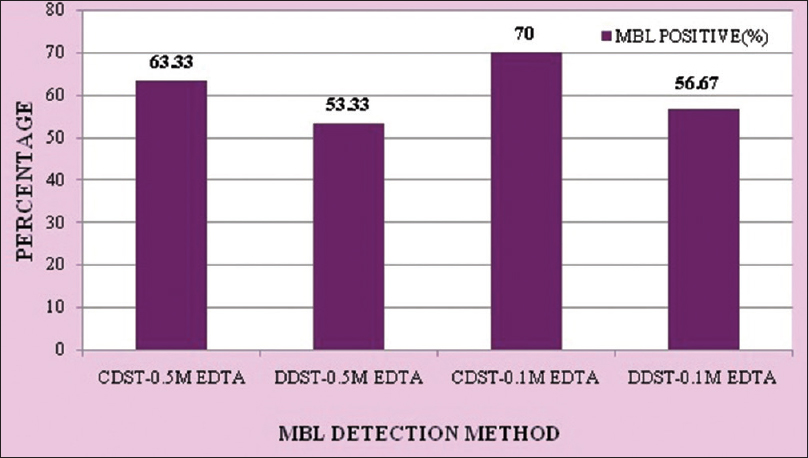
- Comparison of four phenotypic methods for metallo-β-lactamase detection
Prevalence of MBL production was highest in Pseudomonas spp., followed by E. coli, Klebsiella spp., Acinetobacter spp., and P. mirabilis [Table 1].

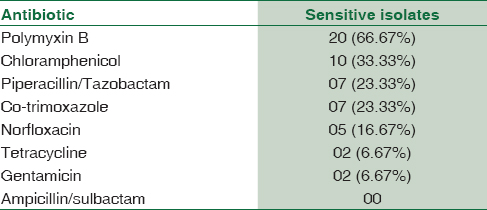
Polymyxin B was found to be the most sensitive drug. Tetracycline and gentamicin were the least sensitive drugs. All MBL-producing Gram-negative Bacilli were resistant to ampicillin/sulbactam [Table 2].
Discussion
MBL-producing Gram-negative organisms have now been reported in many geographic regions.[9] In the present study, prevalence of MBL production in GNB was 19.62%. Other studies have reported 14–62.69% MBL prevalence in GNB.[138]
The emergence of MBL-producing GNB is challenge to microbiology laboratories because there are no standardized guidelines available to detect them.
MBLs spread easily via plasmids, so they rapidly disseminate within an institution and lead to poor outcomes when infection occurs.[101112] The detection of MBL-producing GNB is of crucial importance to limit the spread of MBL gene in between bacteria as well as to early start of appropriate treatment.[13]
Detection of MBL by PCR usually gives reliable and satisfactory results; however, because of the cost the method, it is of limited practical use for routine diagnostic microbiology laboratories. Thus, a simple and inexpensive testing method for detection of MBL producers is necessary.[13]
In the present study, out of four phenotypic methods using EDTA as inhibitor used for confirmation of MBL production, CDST-0.1 M EDTA was found to be the most sensitive (70.00%) method and DDST-0.5M EDTA was found to be the least sensitive (53.33%) method. Franklin et al. have reported 100% sensitivity of CDST-0.1 M EDTA method and 79% sensitivity of DDST-0.1 M EDTA method.[8]
MBL-positive isolates show resistance to all β-lactam antibiotics, aminoglycosides, tetracycline, and fluoroquinolones.[9] In the present study, 21 (66.67%) out of 30 isolates were sensitive to polymyxin B. None of the MBL-producing isolates were sensitive to ampicillin/sulbactam. This antibiotic sensitivity pattern of MBL-positive isolates suggests that early detection of MBL-producing GNB and determination of their antibiotic sensitivity are of crucial importance to start of appropriate treatment.
Conclusion
CDST-0.1 M EDTA was the most sensitive method for MBL detection in GNB. The method is simple to perform, and the materials used are cheap, nontoxic, and easily accessible, making it highly applicable to routine clinical laboratories.
Limitation
In the present study, for confirmation of MBL production, molecular methods were not used.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Detection of metallo betalactamase producing Pseudomonas aeruginosa in hospitalized patients. Indian J Med Res. 2005;122:148-52.
- [Google Scholar]
- Evaluation of phenotypic tests and screening markers for detection of metallo-β-lactamases in clinical isolates of Pseudomonas aeruginosa: A prospective study. Med J DY Patil Univ. 2015;8:599-605.
- [Google Scholar]
- Evaluation of various methods for detection of metallo-β-lactamase (MBL) production in Gram-negative Bacilli. Int J Biol Med Res. 2011;2:775-7.
- [Google Scholar]
- Comparison of phenotypic tests for the detection of metallo-beta-lactamases in clinical isolates of Pseudomonas aeruginosa. Enferm Infecc Microbiol Clin. 2014;32:625-30.
- [Google Scholar]
- Simple screening tests for detection of carbapenemases in clinical isolates of nonfermentative Gram-negative bacteria. Indian J Med Res. 2009;129:707-12.
- [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standard for Antimicrobial Susceptibility Testing; 22nd Informational Supplement M100-S22. Vol 32. Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2012.
- Imipenem-EDTA disk method for differentiation of metallo-β-lactamase-producing clinical isolates of Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol. 2002;40:3798-801.
- [Google Scholar]
- Phenotypic detection of carbapenem-susceptible metallo-β-lactamase-producing gram-negative Bacilli in the clinical laboratory. J Clin Microbiol. 2006;44:3139-44.
- [Google Scholar]
- Metallo-β-lactamases: The quiet before the storm? Clin Microbiol Rev. 2005;18:306-25.
- [Google Scholar]
- Clinical and bacteriological characteristics of IMP-type metallo-beta-lactamase-producing Pseudomonas aeruginosa. Clin Infect Dis. 2003;37:26-32.
- [Google Scholar]
- Population-based epidemiological study of infections caused by carbapenem-resistant Pseudomonas aeruginosa in the Calgary Health Region: Importance of metallo-beta-lactamase (MBL)-producing strains. J Infect Dis. 2005;192:1606-12.
- [Google Scholar]
- Dissemination of the metallo-beta-lactamase gene blaIMP-4 among gram-negative pathogens in a clinical setting in Australia. Clin Infect Dis. 2005;41:1549-56.
- [Google Scholar]
- An evaluation of four different phenotypic techniques for detection of metallo-β-lactamase producing Pseudomonas aeruginosa. Indian J Med Microbiol. 2008;26:233-7.
- [Google Scholar]





