Translate this page into:
Comparison of nonstructural protein-1 antigen detection by rapid and enzyme-linked immunosorbent assay test and its correlation with polymerase chain reaction for early diagnosis of dengue
Address for correspondence: Dr. Sandhya S. Sawant, Room no 303, Third Floor, Department of Microbiology, College building, Nair Hospital, Mumbai Central, Mumbai - 400 008, Maharashtra, India. E-mail: drsandhya.sawant@gmail.com
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
INTRODUCTION:
Early diagnosis of dengue is important for appropriate clinical management and vector control. Different serological tests based on the principle of immunochromatography and enzyme-linked immunosorbent assay (ELISA) are commonly used for detection of antigen and antibodies of dengue virus. The performance of these tests depends on the sensitivity and specificity. Hence, the study was undertaken to compare nonstructural protein-1 (NS1) antigen detection by rapid and ELISA with real-time polymerase chain reaction (RT-PCR) for diagnosis of dengue.
MATERIALS AND METHODS:
Prospective laboratory study was carried out on sera samples (n = 200) from clinically suspected cases of dengue. The sera samples were subjected for NS1 antigen detection test by rapid test, NS1 ELISA, and RT-PCR. The results of rapid and ELISA tests were compared with real Time PCR.
RESULTS:
The sensitivity, specificity, positive, and negative predictive value of rapid dengue NS1 antigen test were 81.5%, 66.7%, 78.2%, and 71.1%, respectively whereas that of NS1 ELISA were 89.9%, 100%, 100%, and 94%, respectively. Concordance of Rapid NS1 and NS1 ELISA with PCR was 75.5% and 94%.
DISCUSSION AND CONCLUSION:
NS1 antigen ELISA can be implemented in diagnostic laboratories for diagnosis of dengue in the acute phase of illness. The test also has great potential value for use in epidemic situations, as it could facilitate the early screening of patients and limit disease expansion.
Keywords
Dengue
NS1 antigen
polymerase chain reaction
rapid and enzyme-linked immunosorbent assay
Introduction
Dengue fever is the rapidly spreading mosquito-borne disease in the world. It has become a major public health concern recently. The infection results in a wide range of symptoms and signs, ranging from inapparent disease to mild undifferentiated fever to more severe and potentially lethal complications in the form of dengue hemorrhagic fever and dengue shock syndrome. The number of cases of severe dengue disease is growing in endemic areas of Southeast Asia, Central and South America, and other subtropical regions. The commonly affected group is children.[12]
Diagnosis of dengue infection in acute phase is important for evidence-based diagnosis, management, implementing control measures, surveillance, and research. The current diagnostic methods for diagnosing dengue is an enzyme-linked immunosorbent assay (ELISA) which measures anti-dengue virus (DENV) IgM or IgG antibodies in patient serum. These antibodies are detectable 4–5 days postsymptom onset (PSO).[1] Number of assays is available in the market for diagnosing dengue during the acute stage of infection. In the year 2000, the first ELISA capable of detecting DENV nonstructural protein-1 (NS1) was developed. NS1 is found in both membrane and soluble forms and is highly conserved.[3] Importantly, NS1 is detectable early during the acute phase (day 1–8 PSO) of both primary and secondary DENV infections. Together, the magnitude and timing of NS1 levels in human clinical specimens makes it an attractive target for various diagnostic assay development.[14] Nowadays, both rapid NS1 (based on immunochromatography principle) and ELISA tests are being used as an effective tool for detection of dengue infection in affected populations. Thus, these tests are useful for both clinical management and vector surveillance.
Since rapid dengue NS1 antigen detection is used in private laboratories and emergency care situations, a study was conducted to compare rapid dengue NS1 antigen detection and NS1 ELISA tests with polymerase chain reaction (PCR) for early diagnosis of DENV infection.
Materials and Methods
A prospective laboratory study was conducted over a period of 6 months from June 2015 to October 2015 (n = 200) in the Department of Microbiology and Molecular Diagnostic Reference Laboratory Associated with Tertiary Care Hospital. Approval of Institutional Ethics Committee was taken before beginning of the study. Before proceeding with any kind of test, informed consent was obtained from the subjects. In case of pediatric patients, assent from the parents was obtained.
Inclusion criteria
-
Clinically suspected cases of dengue presenting with fever from 1 to 9 days along with symptoms such as a headache, myalgia, retro-orbital pain, rash, and hemorrhagic manifestations
-
Patients belonging to age group of 1–80 years.
Exclusion criteria
-
Patients presenting with fever >10 days
-
Patients having a fever with associated focal source of infection (e.g., otitis media, pneumonia, meningitis), coryza, chronic illness, including anemia, or unstable vital signs.
Type of sample and collection
Approximately, 3–5 ml of blood sample was collected aseptically from clinically suspected dengue fever patients after written informed consent and/or assent in plain and ethylenediaminetetraacetic acid vacutainer. In addition, relevant demographic, clinical, and investigational information was collected from patient's record file. Detailed clinical history of the patient with relevant records was recorded in case record form.
Sample processing
Collected samples were centrifuged at 2500 rpm for 15 min to obtain serum and plasma. The sera were subjected to rapid dengue NS1 antigen test, and NS1 ELISA, and plasma samples were used for real-time RT-PCR.
Rapid dengue nonstructural protein-1 antigen detection test
In all, 200 samples were tested by rapid dengue NS1 antigen detection test based on the principle of immunochromatography (Manufacturer – Bhat Bio-Scan). The manufacturer's instructions were followed in the procedure, and the results were interpreted as positive or negative.
Dengue nonstructural protein-1 antigen detection enzyme-linked immunosorbent assay
NS1 antigen ELISA was performed on all sera samples (n = 200). The kit used was NS1 antigen RecombiELISA by CTK Biotech. The manufacturer's instructions were strictly followed for performing the test and interpreting the results. The O. D. was measured at 450 nm using ELISA reader.
Real time RT-polymerase chain reaction
RNA extraction was done using automated MagNA Pure 96, (Roche System) for viral RNA extraction from samples. The extracted nucleic acid elutes from patients sample was then subjected to RT-PCR and the following probes and primers were used. Samples were subjected to PCR conditions as per the manufacturer's instructions. PCR products were detected in real-time on a LightCycler instrument using 5’-nuclease technology. PCR results were obtained within 90 min (55 cycles and melting curve) [Table 1].

Statistical methods
The entire data were entered and cleaned in MS Excel before its statistical analysis. All the results are shown in tabular and graphical format, wherever necessary to visualize the statistically significant difference more clearly. The P < 0.05 was considered to be statistically significant. All the hypotheses were formulated using two-tailed alternatives against each null hypothesis (hypothesis of no difference). The entire data were analyzed statistically using Statistical Package for Social Sciences (SPSS version 11.5, Inc., Chicago, IL, USA) for MS Windows. Assessment of sensitivity, specificity, positive and negative predictive values and concordance were calculated.
Results
Fever (98.5%) and chills (100%) were the most common clinical manifestations found [Figure 1]. The median age of the study population was 22.7 years, and sex ratio (male:female) was 1.4:1. Out of 200 samples subjected to the different test, RT-PCR was positive in 119 (59.5%) and negative in 81 (40.5%) for DENV. NS1 antigen ELISA was positive in 107 (53.5%) samples and negative in 93 (46.5%) and rapid dengue NS1 antigen detection test was positive in 124 (62%) and negative in 76 (38%) samples.
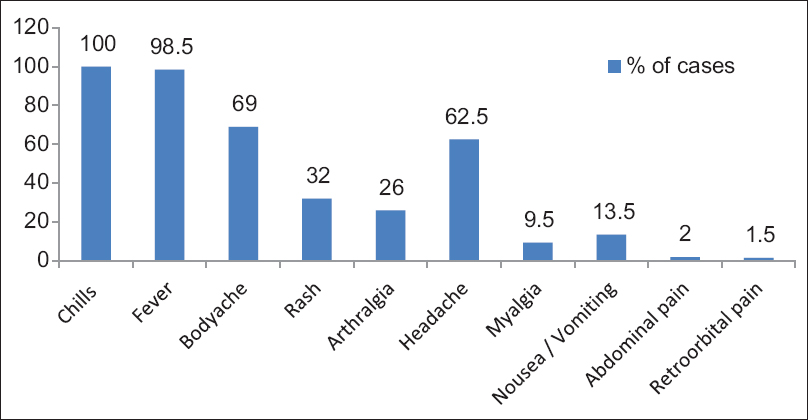
- Clinical profile of the patients
Comparison of rapid and dengue nonstructural protein-1 enzyme-linked immunosorbent assay with real-time RT polymerase chain reaction as gold standard
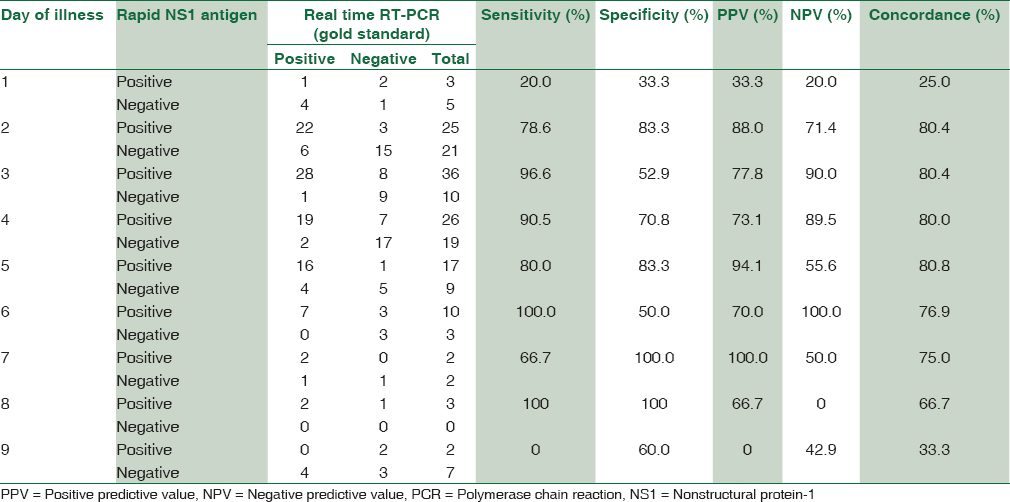
The sensitivity and specificity of rapid dengue NS1antigen detection test were found to be 81.5% and 66.7%, respectively. Positive predictive value and negative predictive value were 78.2% and 71.1%, respectively. Concordance of rapid dengue NS1 detection with real-time RT-PCR was found to be 75.5% [Figure 2]. Maximum concordance was seen on day 2–5, i.e., 80% [Table 2].
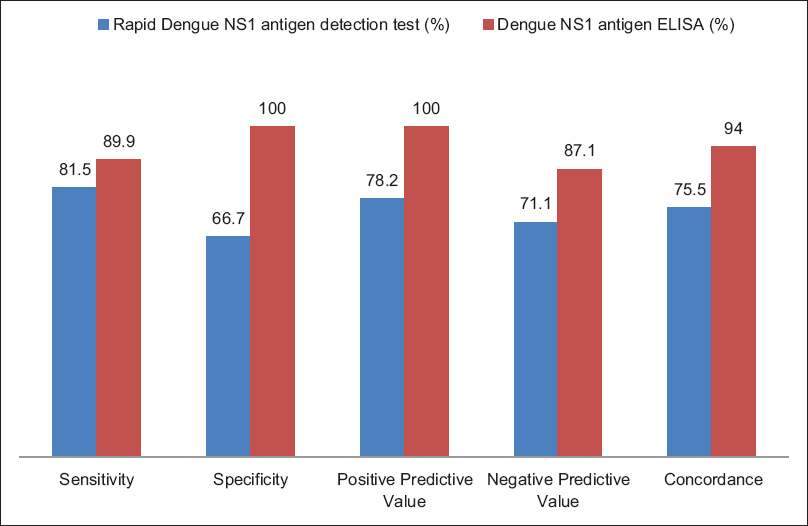
- Performance of rapid and dengue nonstructural protein-1 enzyme-linked immunosorbent assay with real-time RT polymerase chain reaction as gold standard
The sensitivity and specificity of dengue NS1 antigen ELISA were found to be 89.9% and 100%, respectively. Positive predictive value and negative predictive value were 100% and 87.1%, respectively. Concordance of NS1 antigen ELISA with real-time PCR was found to be 94% with a maximum concordance of 100% from day 3 to 8 [Figure 2 and Table 3].
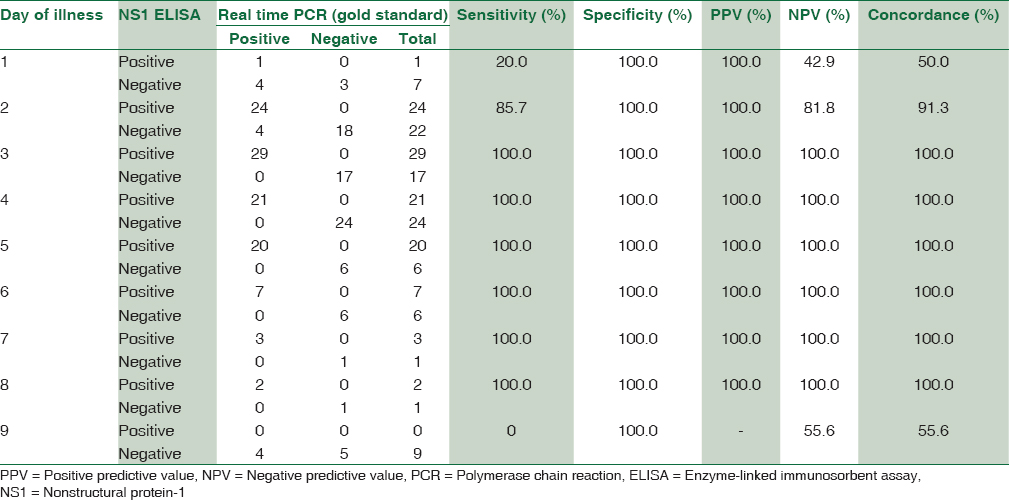
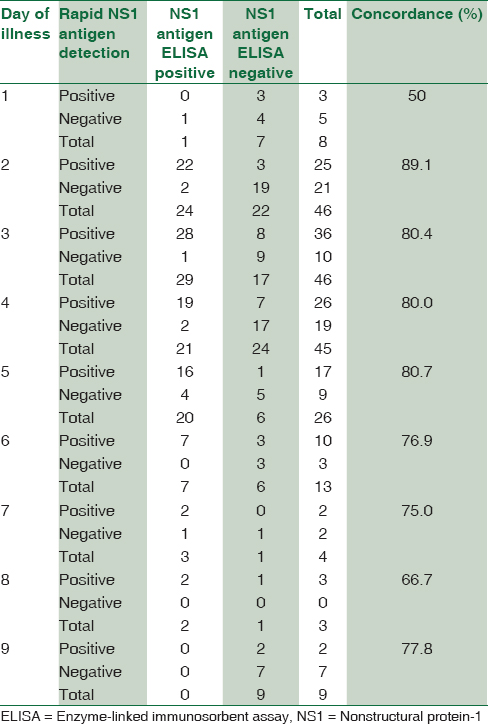
Concordance between rapid dengue nonstructural protein-1 antigen detection test and nonstructural protein-1 antigen enzyme-linked immunosorbent assay on different days of illness
A good concordance was found between rapid NS1 antigen detection and NS1 antigen ELISA from day 2 of illness. A maximum concordance of about 89.1% was seen on day 2 of illness followed by day 3–5 showing a concordance of almost 80% [Table 4].
Discussion
Patients with acute febrile illness (AFI) have a differential diagnosis of dengue, leptospirosis, malaria, enteric fever, and other viral infections. Of all these, dengue is an important cause of high morbidity and mortality in India. The current standard of care includes diagnosis of dengue by antigen and antibody tests. Tests for antigen and antibody detection are widely used for confirmation of dengue viral infection. However, the performance of tests depends on specificity and sensitivity of test kits used.
Therefore, an accurate detection of DENV infection from acute phase viraemic blood samples in patients with AFI contributes greatly to evidence-based diagnosis and patient management in hospitals and promotes vector control measures by the public health department.
Our study conducted over a period of 6 months from June 2015 to October 2015 (n = 200). The clinical profile in the study revealed that fever and fever with chills were the most common presenting symptom (98.5% and 100%, respectively). Fever was seen in all patients who were studied. Neeraja et al. (100%) and Shah et al. (100%) have also substantiated fever as being the most common presenting symptom. However, Ghosh et al. from Mumbai in 2013 have reported 71% of patients presenting with fever with chills.[567]
In the present study, we compared the rapid dengue NS1 antigen detection test and NS1 ELISA test with real-time RT-PCR for early diagnosis of dengue. The sensitivity and specificity of rapid dengue NS1 antigen detection test were found to be 81.5% and 66.7%, respectively. Whereas the sensitivity and specificity of NS1 antigen ELISA was found to be 89.9% and 100%, respectively. Studies conducted across India have found sensitivity and specificity within a similar range. Hunsperger et al. have reported NS1 rapid diagnostic test sensitivity as 38%–71% and specificity 76%–80%.[8] Shrivastava et al. in 2011 reported rapid tests sensitivity, specificity, positive predictive value, and negative predictive value of 62.5%, 100%, 100%, and 88.15%, respectively.[9] Ahmed and Broor in 2014 have reported the sensitivity, specificity, PPV, and NPV of NS1 antigen ELISA to be 73.53%, 100%, 100%, and 70%, respectively.[3] Hunsperger et al. reported NS1 antigen ELISA sensitivity to be 60%–75% and specificity 71%–80%.[8] Published literature mentions sensitivity of NS1 antigen ELISA ranging from 76% to 97% and specificity from 98% to 100%.[101112]
The sensitivity of rapid NS1 antigen detection test on day 3 of fever was 96.6%, day 4 was 90.5%, and day 5 was 80% in our study. It was also observed that on day 6 and 8 a sensitivity of 100% was seen, but we had only 13 and 3 patients enrolled with day 6 and 8 of fever, respectively which becomes a limiting factor, with insignificant statistical distribution. Day 1 also had the least sensitivity and concordance with PCR, i.e. 20% and 25% with only 8 patients presenting with day 1 of fever which is again a statistically insignificant number to come to the conclusion.
In this study, the concordance of Rapid dengue NS1 detection with real-time RT-PCR was found to be 75.5%. Concordance with PCR in a study by Hunsperger et al. was found to be 90.1%.[8] The concordance between NS1 antigen ELISA and real-time RT-PCR in our study was found maximum, i.e., 100% from day 3 to 8. The sensitivity of NS1 antigen ELISA was 20% on day 1 as only 8 patients presented with day 1 of fever which is statistically insignificant number to come to a conclusion. Ahmed and Broor reported concordance between NS1 antigen ELISA and RT-PCR to be 75% on day 1, 83.3% on day 2, 92.9% on day 3, 75% on day 4, and 50% on day 5 which is correlating with our study.[3]
In the present study, we found that the sensitivity and specificity of rapid dengue NS1 antigen test were 81.5% and 66.7% whereas that of NS1 ELISA was 89.9% and 100%, respectively. In published literature, the sensitivity of NS1 antigen detection by ELISA ranges between 76%–97% and specificity, 98%–100%. The overall performance of NS1 antigen ELISA was found to be better than rapid dengue NS1 antigen detection in our study, in terms of sensitivity and specificity. Furthermore, a higher detection rate by both rapid and NS1 LISA tests was found from day 3 of illness.
With the increase of the geographic range of dengue fever and the increasing number and severity of reported cases all over the country, the use of NS1 antigen detection and real-time RT-PCR could allow clinical diagnostic laboratories to identify DENV infections early enough to adjust patient management, reducing the time between detection of the first cases, and the notification to public health authorities, facilitating vector control measures.[3131415]
Conclusion
NS1 antigen ELISA can be implemented in diagnostic laboratories for diagnosis of dengue in the acute phase of illness. The test also has great potential value for use in epidemic situations, as it could facilitate the early screening of patients and limit disease expansion. NS1 antigen ELISA and real-time RT-PCR are valuable techniques for rapid and early diagnosis of dengue.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Dengue/dengue hemorrhagic fever: The emergence of a global health problem. Emerg Infect Dis. 1995;1:55-7.
- [Google Scholar]
- Comparison of NS1 antigen detection ELISA, real time RT-PCR and virus isolation for rapid diagnosis of dengue infection in acute phase. J Vector Borne Dis. 2014;51:194-9.
- [Google Scholar]
- World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control, New Edition 2009. A Joint Publication of the World Health Organization (WHO) and the Special Programme for Research and Training in Tropical Diseases (TDR) 2009
- Serodiagnosis of dengue virus infection in patients presenting to a tertiary care hospital. Indian J Med Microbiol. 2006;24:280-2.
- [Google Scholar]
- Clinical and laboratory profile of dengue infection in children. Kathmandu Univ Med J (KUMJ). 2006;4:40-3.
- [Google Scholar]
- A Clinicomicrobiological Study of dengue fever cases in a tertiary care centre of Navi Mumbai. Int J Bioassays. 2013;2:1462-7.
- [Google Scholar]
- Evaluation of commercially available diagnostic tests for the detection of dengue virus NS1 antigen and anti-dengue virus IgM antibody. PLoS Negl Trop Dis. 2014;8:e3171.
- [Google Scholar]
- Evaluation of a commercial dengue NS1 enzyme-linked immunosorbent assay for early diagnosis of dengue infection. Indian J Med Microbiol. 2011;29:51-5.
- [Google Scholar]
- Enzyme-linked immunosorbent assay specific to dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J Clin Microbiol. 2002;40:376-81.
- [Google Scholar]
- Potential application of nonstructural protein NS1 serotype-specific immunoglobulin G enzyme-linked immunosorbent assay in the seroepidemiologic study of dengue virus infection: Correlation of results with those of the plaque reduction neutralization test. J Clin Microbiol. 2002;40:1840-4.
- [Google Scholar]
- High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J Infect Dis. 2002;186:1165-8.
- [Google Scholar]
- National guidelines for diagnosis and management of dengue fever/dengue haemorrhagic fever and dengue shock syndrome. J Indian Med Assoc. 2011;109:30-5.
- [Google Scholar]
- Comparison of two commercially available dengue virus (DENV) NS1 capture enzyme-linked immunosorbent assays using a single clinical sample for diagnosis of acute DENV infection. Clin Vaccine Immunol. 2008;15:1513-8.
- [Google Scholar]
- Evaluation of an NS1 antigen detection for diagnosis of acute dengue infection in patients with acute febrile illness. Diagn Microbiol Infect Dis. 2008;60:387-91.
- [Google Scholar]





