Translate this page into:
Deciphering the Patterns of Dual Primary Cases Registered at the Hospital-Based Cancer Registry: First Experience from Rural Cancer Center in North India
Address for correspondence: Atul Budukh, PhD, TataMemorial Centre (TMC), Homi Bhabha National Institute, (HBNI), Centre for Cancer Epidemiology, ACTREC, Sector 22, Utsav Chowk, CISF Road, Kharghar, Navi Mumbai, Maharashtra 410 210, India (e-mail: atul.budukh@gmail.com; budukham@tmc.gov.in).
-
Received: ,
Accepted: ,
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives
The objective is to present the patterns of dual primary malignancies diagnosed at the Pathology Laboratory of Cancer Hospital with the support from hospital-based cancer registry (HBCR), Sangrur, Punjab, India for the years 2018 and 2019.
Methods
HBCR abstracts data from electronic medical records. Trained cancer registry staff abstracts cases in standard pro forma. Dual primary was coded as per the International Agency for Research on Cancer rule and was rechecked by the pathologist.
Statistical Analysis
Data about multiple primary was entered and documented in an Excel sheet. Time interval was calculated by subtracting the date of diagnosis for second primary and first primary.
Results
A total of 6,933 cases were registered, 45 cases are dual primary (26 females, 19 males) of which 64.4% are synchronous and 35.6% metachronous cases. Seventy-nine percent received cancer-directed treatment for synchronous and 87% for metachronous. The most common sites of the primary tumor were breast (33%), head and neck (22.2%), gynecological sites (11%), prostate (9%), esophagus (4%), and remaining other tumors (20.8%). Most common sites for second malignancies were gastrointestinal (GI) tract (31%), gynecological sites (18%), head and neck (16%), hematological malignancies (7%), soft tissue sarcoma (4%), breast (2%), and other sites (22%).
Conclusion
More than 70% of cases of primary tumors were in breast, head and neck, gynecological, and prostate. Of these, more than 60% of the second malignancy was found in the GI tract, gynecological, and head and neck sites. Around two-thirds of dual tumors are synchronous. Breast cancer cases have higher incidence of second malignancy. Regular follow-up is necessary to assess the survival of the second primary.
Keywords
Dual primary
synchronous
hospital-based cancer registry
metachronous
Introduction
Globally, the projected cancer burden for the year 2040 is expected to rise from 19.3 million to 28.4 million, as reported by GLOBOCAN 2020.[1,2] Over the last few decades, advancements in cancer diagnostics and treatments have resulted in better early cancer detection and disease management, and eventually, higher survival.[3] With increased numbers of cured patients and long-term survival, the risk of developing multiple primary malignant tumors is increasing.[4] The incidence of dual primary malignancy has not been rare at all.[5,6] Based on an analysis of several studies, the incidence of multiple primary malignant neoplasm (MPMN) was estimated to be 0.73 to 11.7% in the literature with widely disparate prevalence estimates reported.[7] Warren and Gates established the criteria for diagnosing double primary tumors, which were further revised. For MPMN, the diagnostic criteria are as follows: first, all tumors must be malignant as determined by histopathology; second, each must be geographically distinct and unique, with normal mucosa used to distinguish the lesions; and third, the possibility that one is a metastasis of the other must be ruled out.[8–11] MPMN is defined as two or more unrelated primary malignant tumors that originate from different organs and occur in the body at the same time or one after another.[7] If the tumors occur simultaneously or within 6 months of one another then it is accepted as synchronous (sMPMN), and if the interval time is more than 6 months then it is accepted as metachronous (mMPMN).[12] Different factors are associated with an increased risk of developing more than one primary cancer including genetic susceptibility and familial cancer syndromes, environmental and lifestyle exposures (e.g., tobacco, alcohol use), hormonal factors, immune deficiency and infection, carcinogenic effects of prior cancer treatments, and finally, interaction among all of these factors. Diagnosis and treatment for multiple cancers remain a challenge because of variable definitions of multiple primaries, the lack of specific screening guidelines, and the lack of well-established treatment guidelines.[13] MPMNs can be often confusing whether it is recurrence or distant metastasis of primary tumors since both are characterized by new lesions. Metastatic tumors are derived from the primary lesion, with both showing the same pathological characteristics and similar developmental processes and prognosis. In literature, data regarding the occurrence and the outcome of MPMNs from the Indian subcontinent are limited, apart from that the practical implications of the management of patients with multiple primaries are rarely discussed.[14] In a patient with previous cancer history and potentially prior anticancer therapy, it can be difficult to establish the diagnosis of an additional primary and when a patient with two active malignancies is diagnosed at the same time.[13] The challenge is to find an anticancer therapy strategy that covers both cancer types without increased toxicity or relevant pharmacological interactions and negative impact on the overall outcome.
Because of an increased MPMN occurrence, we aimed to evaluate the clinical characteristics, diagnosis, and treatment delivered to MPMN patients retrospectively who registered at our institution in 2 years. In the present study, we aim to study the epidemiological pattern of dual primary malignancies observed in patients. The Homi Bhabha Cancer Hospital (HBCH), Sangrur, Punjab, India is functional since January 2015. The hospital provides holistic diagnostic facilities such as computerized tomography scan, magnetic resonance imaging, ultrasonography, mammography, biochemistry, hematology, tumor marker, histopathology, immunohistochemistry, and cytology. Additionally, the hospital provides surgical, radiotherapy, and medical oncology services based on the treatment protocol provided by Tata Memorial Centre (TMC), Mumbai, Maharashtra, India. The hospital also provides preventive services in Sangrur like early detection of breast, cervix, and oral cancer. This hospital has both population-based and hospital-based cancer registries.[15,16]
Materials and Methods
This is a retrospective collection of data from a hospital-based cancer registry (HBCR) running in the HBCH, Sangrur. The registry records information through the electronic medical record of the hospital. Extensive case abstraction of patients diagnosed with second de novo malignancy was done by trained HBCR staff. The study follows International Agency for Research on Cancer (IARC) rules. All the sociodemographic as well as clinical information such as age at diagnosis of each tumor, sex, whether synchronous or metachronous, site of origin, method of diagnosis, histology, stage at presentation of disease, and treatment, have been recorded in a predesigned pro forma. The primary site and histology were coded using the International Classification of Diseases for Oncology 3rd Edition (ICD-O3).[17] Abstracted data of cancer cases were regularly entered in the CanReg5 software designed by IARC, World Health Organization.[18]
Patients with histologically/radiologically proven synchronous or metachronous dual primaries as defined by Warren and Gates criteria, over 2 years (2018 and 2019) were included in the study. The time interval to differentiate between synchronous or metachronous has been taken as 6 months as reported by several authors.[19–21] We have excluded patients without any histological/radiological evidence of each tumor and the patients in whom, the second tumor was suspected to be a metastasis of the first location.[12]
The entered data were checked by the senior staff from the Centre for Cancer Epidemiology–TMC (CCE-TMC) for quality control. Any error observed in the case abstraction was discussed with the clinician and registry staff. Moreover, for the accuracy of the data, CCE-TMC staff randomly checked the data through the TMC server and senior staff visited Sangrur to discuss the errors with concerned staff and made sure that the errors were corrected and reentered carefully into the database. The final data entered was analyzed using the CanReg5 software and SPSS software version 21.0 (IBM, Armonk, New York, United States). The detailed method has been described in our previous research article.[22]
Results
As shown (►Fig. 1) over 2 years (2018–2019), 6,933 patients were registered to the HBCH, Sangrur (2,969 in 2018 and 3,964 in 2019). Out of 6,933 registered cases, 5,819 (84%) were diagnosed with malignancy; 2,521 (43%) in 2018 and 3,298 (57%) in 2019. Out of total registered malignant cases, 45 (10 for the year 2018 and 35 for the year 2019) dual primary malignancies were observed which is 0.8% of total malignant cases registered in HBCH, Sangrur during the 2 years. Among 45 dual primary cases, 29 (64.4%) were synchronous (►Table 1) and 16 (35.6%) were metachronous (►Table 2). It was found that 44.4% of dual primary cases were from district Sangrur followed by Patiala (20%), Ludhiana (11.1%), 4.4% each from Amritsar, Barnala, Fatehgarh Sahib, and Mansa, and 6.6% cumulatively (2.2% each) from Bathinda, Fazilka, and Kaithal districts.
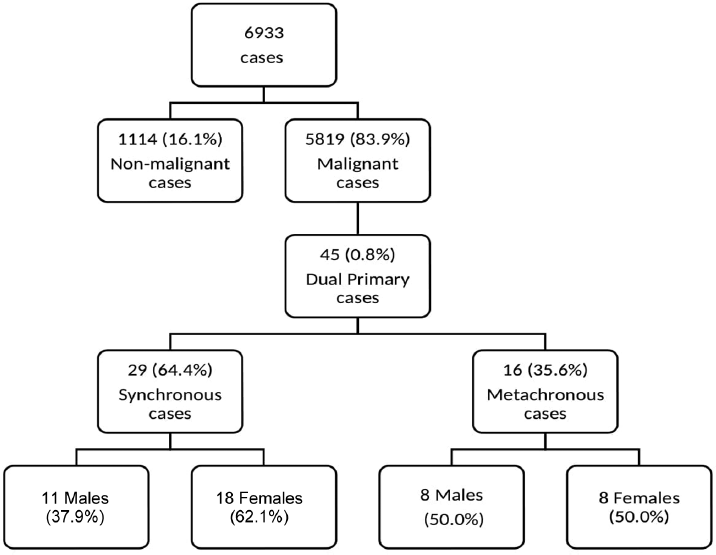
- Flowchart of distribution of cases identified in hospital-based cancer registry (HBCR).
| Age at primary/ Sex | Primary | Histology | Treatment | Second site | Age at second malignancy | Histology | Time interval (in months) | Treatment | District |
|---|---|---|---|---|---|---|---|---|---|
| 70/F | Breast | IDC | Surgery + CT | Liver | 70 | HCC | 0.6 | Surgery | Sangrur |
| 49/F | Breast | IDC | Surgery + CT + RT | Kidney | 49 | Clear cell carcinoma | 1.5 | Surgery | Fatehgarh Sahib |
| 52/F | Breast | IDC | Surgery + Ct + RT | Jejunum | 52 | Carcinoid tumor | 4.9 | Surgery + CT + RT | Sangrur |
| 51/F | Breast | IDC | Surgery + CT + RT | Thyroid | 51 | Papillary carcinoma | 1.5 | Surgery | Ludhiana |
| 36/F | Breast | IDC | CT | Rectum | 37 | Adenocarcinoma | 4.2 | CT + RT | Mansa |
| 62/F | Breast | IDC | Surgery + CT | Gallbladder | 62 | Adenocarcinoma | 0.06 | Surgery | Sangrur |
| 49/F | Breast | IDC | Surgery + CT | Ovary | 49 | Serous adenocarcinoma | 2.9 | Surgery + CT | Sangrur |
| 48/F | Breast | IDC | Surgery + CT + RT | Kidney | 49 | Renal cell carcinoma | 3.6 | Surgery | Sangrur |
| 56/F | Breast | IDC | Surgery + CT + RT | Stomach | 56 | GIST | 0.3 | Observe | Ludhiana |
| 66/M | Hypopharynx | Basaloid SCC | Not treated at HBCH (treated outside) | Esophagus | 66 | Adenocarcinoma | 2 | Not treated at HBCH (treated outside) | Sangrur |
| 41/F | Hypopharynx | SCC | CT + RT | Kidney | 41 | Clear cell carcinoma | 2.9 | Not treated at HBCH | Sangrur |
| 59/M | Larynx | SCC | CT + RT | Floor of mouth | 59 | SCC | 0 | CT + RT | Mansa |
| 38/M | Larynx | SCC | CT + RT | Bladder | 38 | Papillary transitional cell carcinoma | 2 | CT + RT | Barnala |
| 51/M | Larynx | SCC | RT | Esophagus | 51 | SCC | 0.2 | CT + RT | Ludhiana |
| 77/M | Oropharynx | SCC | RT | Hypopharynx | 77 | SCC | 0.9 | RT | Patiala |
| 64/M | Thyroid | Medullary carcinoma | Treatment advised not taken | Bladder | 64 | Papillary transitional cell carcinoma | 1.3 | CT | Fatehgarh Sahib |
| 68/M | Tongue | SCC | Treatment advised not taken | Thyroid | 68 | Papillary carcinoma | 1.7 | Treatment advised not taken | Sangrur |
| 64/F | Tongue | SCC | Not treated at HBCH (treated outside) | Lymph node | 64 | Small lymphocytic lymphoma | 1.1 | Not treated at HBCH (patient defaulted) | Patiala |
| 51/F | Cervix | SCC, keratinizing | CT + RT | Lymph node | 51 | NHL-DLBCL | 1.3 | CT + RT | Sangrur |
| 65/F | Ovary | Serous adenocarcinoma | Not treated at HBCH (patient defaulted) | Breast | 65 | IDC | 0.2 | Not treated at HBCH (patient defaulted) | Sangrur |
| 73/F | Ovary | Serous adenocarcinoma | Not treated at HBCH (treated outside) | Lung | 73 | Adenocarcinoma | 0.9 | CT | Patiala |
| 76/F | Ovary | Serous adenocarcinoma | Surgery + CT | Gallbladder | 77 | Adenocarcinoma | 3.5 | Surgery | Sangrur |
| 70/M | Prostate | Adenocarcinoma | CT | Bone marrow | 70 | Polycythemia vera | 3.2 | Not treated at HBCH | Patiala |
| 41/F | Esophagus | Neoplasm Malignant | CT | Cervix | 41 | SCC, non-keratinizing | 3.6 | CT + RT | Sangrur |
| 64/F | Esophagus | SCC | CT + RT | Oropharynx | 64 | SCC | 0 | CT + RT | Patiala |
| 58/M | Bladder | Transitional cell carcinoma | CT + RT | Lung | 58 | SCC | 1.5 | CT + RT | Sangrur |
| 70/F | Anal canal | SCC | RT | Cervix | 70 | SCC, non-keratinizing | 0.7 | RT | Barnala |
| 58/M | Rectum | Signet ring cell carcinoma | CT + RT | Prostate | 58 | Adenocarcinoma | 2.5 | CT | Patiala |
| 74/M | Lymph node | NHL-DLBCL | CT | Tongue | 74 | SCC | 0 | CT | Amritsar |
Abbreviations: CT, chemotherapy; F, female; GIST, gastrointestinal stromal tumor; HBCH, Homi Bhabha Cancer Hospital; HCC, hepatocellular carcinoma; IDC, infiltrated duct carcinoma; M, male; NHL-DLBCL, non-Hodgkin-lymphoma diffuse large B-cell; RT, radiotherapy; SCC, squamous cell carcinoma.
| Age at primary/ Sex | Primary | Histology | Treatment | Second site | Age at second malignancy | Histology | Time interval (in years) | Treatment | District |
|---|---|---|---|---|---|---|---|---|---|
| 65/F | Breast | IDC | Surgery | Soft tissue | 68 | Fibrosarcoma | 3 | Surgery | Sangrur |
| 51/F | Breast | IDC | Surgery + CT + RT (treated outside) | Ovary | 60 | Serous adenocarcinoma | 9 | CT + RT | Amritsar |
| 51/F | Breast | Neoplasm malignant | Surgery (treated outside) | Ovary | 58 | Serous adenocarcinoma | 7 | Surgery + CT | Ludhiana |
| 50/F | Breast | Neoplasm malignant | Surgery + CT (treated outside) | Endometrium | 58 | Endometrioid adenocarcinoma | 8 | Not treated at HBCH (patient defaulted) | Bathinda |
| 29/F | Breast | Neoplasm malignant | Surgery + HT (treated outside) + RT (at HBCH) | Ovary | 36 | Neuroendocrine carcinoma | 7 | CT (outside) + RT (at HBCH) | Sangrur |
| 69/F | Breast | Neoplasm malignant | BSC | Soft tissue | 76 | Angiomyosarcoma | 7 | BSC | Fazilka |
| 54/F | Cervix | Neoplasm malignant | CT + RT (treated outside) | Ovary | 65 | Serous adenocarcinoma | 11 | CT + RT | Sangrur |
| 60/M | Prostate | Adenocarcinoma | Surgery + HT (treated outside) | Anal canal | 64 | SCC | 4 | CT + RT | Ludhiana |
| 78/M | Prostate | Adenocarcinoma | HT + RT | Gallbladder | 79 | Adenocarcinoma | 1 | BSC | Sangrur |
| 61/M | Bladder | Neoplasm malignant | Surgery (treated outside) | Bone marrow | 73 | Chronic lymphocytic leukemia | 12 | Under observation at HBCH | Sangrur |
| 71/M | Kidney | Neoplasm malignant | Surgery (treated outside) | Bone marrow | 71 | Multiple myeloma | 0.75 | Treatment advised not taken | Patiala |
| 46/F | Rectum | Adenocarcinoma | Surgery (treated outside) + CT + RT (at HBCH) | Thyroid | 49 | Papillary microcarcinoma | 3 | Surgery | Sangrur |
| 51/M | Ampulla of Vater | Adenocarcinoma | Surgery + CT + RT (treated outside) | Colon | 52 | Adenocarcinoma | 1 | Surgery | Patiala |
| 62/M | Retromolar area | SCC | Surgery | Liver | 63 | Neoplasm malignant | 7.1 | No treatment | Sangrur |
| 62/M | Prostate | Neoplasm malignant | Not treated at HBCH (patient defaulted) | Bladder | 62 | Neoplasm malignant | 8 | Not treated at HBCH (patient defaulted) | Kaithal |
| 53/M | Brain | Glioblastoma | Surgery + CT + RT | Prostate | 55 | Adenocarcinoma | 1.2 | CT | Patiala |
Abbreviations: BSC, best supportive care; CT, chemotherapy; F, female; HBCH, Homi Bhabha Cancer Hospital; HT, hormone therapy; IDC, infiltrated duct carcinoma; M, male; RT, radiotherapy; SCC, squamous cell carcinoma.
Of the 45 cases, 26 (57.8%) were females and 19 (42.2%) were male cases. The median age for both synchronous was 59 and metachronous malignancies were 57. The most common site of the primary tumor (►Fig. 2) was breast (15 cases; 33.3%) followed by head and neck (H&N) cancer (10 cases; 22.2%), gynecological cancer (5 cases; 11.1%), prostate cancer (4 cases; 8.9%), esophagus cancer (2 cases; 4.4%), and other sites (9 cases; 20.0%) which are also in the top leading sites in males and females in HBCR.[22]
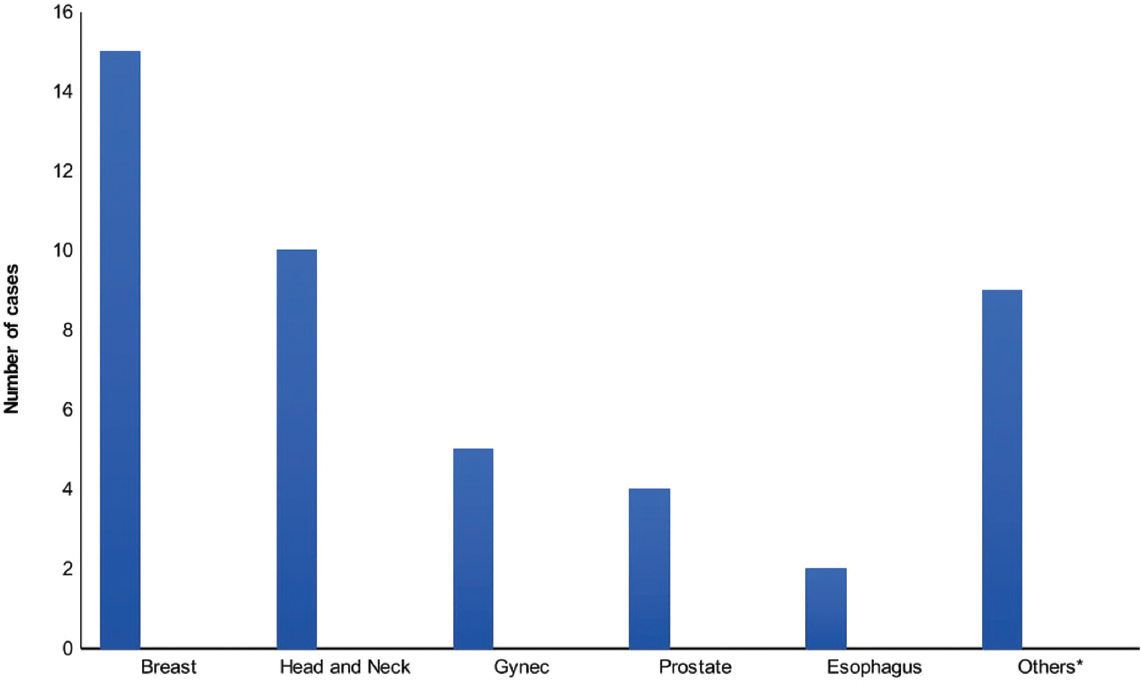
- Site distribution of primary malignancies.
The most common site for second malignancy (►Fig. 3) was the gastrointestinal tract (GI tract) (14 cases; 31.1%) followed by gynecological cancer (8 cases; 17.8%), H&N (7 cases 15.6%), hematological (3 cases; 6.7%), soft tissue sarcoma (STS) (2 cases; 4.4%), breast (1 case; 2.2%), and other sites (10 cases; 22.2%). The minimum and maximum age of diagnosis for the second primary was found to be 36 and 79 years, respectively. The average age difference between primary and second malignancies was found to be 4.7 years in metachronous cases.
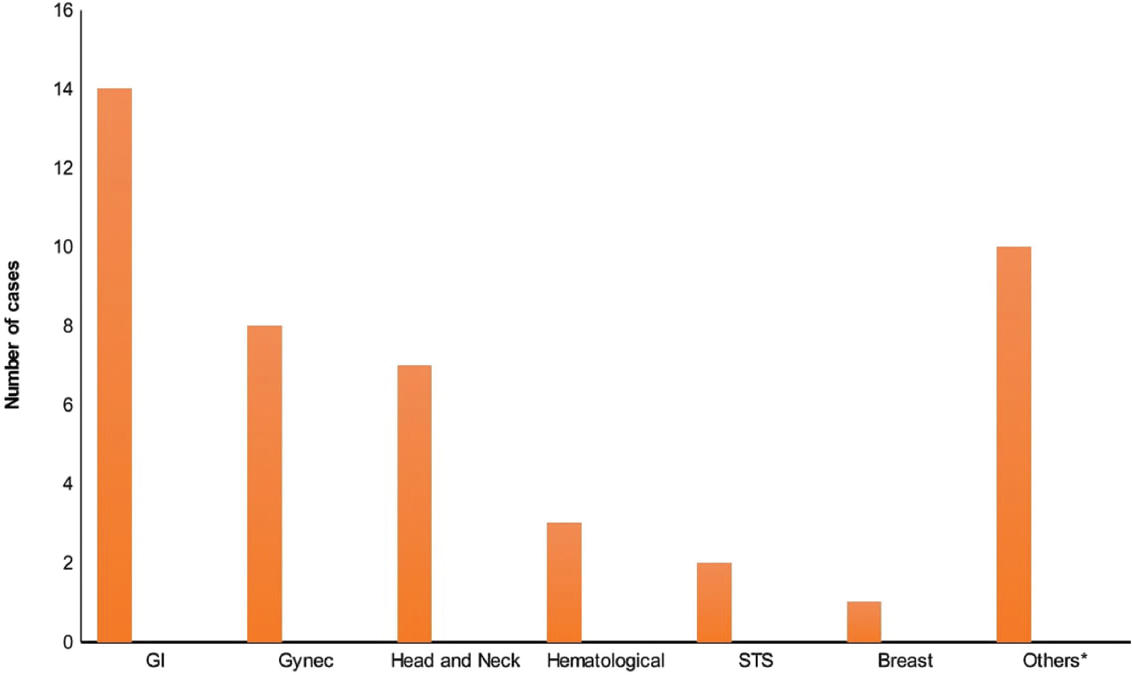
- Site distribution of second malignancies.
It was also observed that out of 15 primary breast cancers, the second tumor was developed in 5 cases of gynecological sites followed by GI tract (5 cases), STS (2 cases), urinary tract (2 cases), and H&N (1 case). Out of 10 primary H&N cases, 3 occurred in H&N for second malignant neoplasm, 2 in the bladder, 1 in the urinary tract, 2 in the esophagus, 1 in the GI tract, and 1 in the lymphatic system. Out of 5 primary gynecological cases, 1 presented in the gynecological region as a second malignancy, 1 in the breast, 1 in the lung, 1 in the gallbladder, and 1 in the lymphatic system. In the same manner, out of 4 primary prostate cancers, a second malignancy appeared in the GI tract (2 cases), hematological cancers (1 case), and bladder (1 case). Similarly, out of 2 primary esophagus cancer cases, 1 case presented in gynecological sites, and another one was found in H&N as a second malignancy.
Out of 29 synchronous dual primaries, 23 cases (79.3%) received cancer-directed treatment (CDT) for the first malignancy while 22 cases (75.9%) got treated for the second primary at HBCH, Sangrur. Overall, 20 (68.9%) patients received the treatment for both synchronous malignancies. It is observed that, out of 20 treated cases, 9 (45%) were from Sangrur, 3 (15%) from Patiala, 2 (10%) from Mansa, 2 (10%) from Barnala, and 4 (20%) were from other districts which are situated more than 80 km from HBCH. On the other hand, out of 16 metachronous cases, 14 (87.5%) patients have received CDT for primary (10 treated outside of HBCH and 4 treated at HBCH) and 9 patients received treatment at HBCH for the second primary. Out of 9 treated cases, 4 (44.4%) were from Sangrur, 2 (22.2%) from Patiala, and 3 (33.3%) were from other districts which are situated more than 80 km from HBCH.
Discussion
For the first time, data have been presented on dual primary cases from a rural tertiary cancer hospital in Punjab, from North India which is a unit of TMC in Mumbai, India. HBCH Sangrur has a competent diagnostic and treatment infrastructure that is accessible and provides benefits of various financial support schemes by the state and central government.[23] Among the total cancer patients who were registered to the HBCH, Sangrur in 2018 and 2019, the female-to-male ratio was 1.19:1, while the female-to-male ratio for double primary malignancies was found to be 1.37:1 which is in good agreement with the female patients registered to the hospital. These data results are following the study results about breast cancer among females indicating they have a slightly higher chance to develop a second malignant neoplasm.[24] Although the mechanisms underlying the emergence of MPMNs have not been fully explained, among the most common factors that have been implicated are genetic predisposition, the immune system of patients, and extensive exposure to carcinogens, which include chemo- and/or radiotherapy used in tumor treatment.[12] However BRCA1 and BRCA2 mutations as well as obesity may be responsible for the higher number of dual primary breast malignancies.[25] Similarly, patient with H&N squamous cell carcinoma (HNSCC) are at elevated risk of second primary malignancies, most commonly of the H&N, lung, and esophagus and it may be due to association of human papilloma virus H&N sites like oropharyngeal cancers.[21]
Our results are in good agreement with the average time duration which is around 5 years to develop a second malignant neoplasm as reported by Kim and Song.[26] Similar results were reported by some past studies carried out by Bagri et al, Suzuki et al, and Sharma et al.[12,19,27] A study conducted in a European cohort shows women surviving breast cancer have a 30% increased risk of developing endometrial, colorectal, lymphoma, melanoma, and kidney cancers which are best reflected in primary breast cancer cases as reported by us as 33% second malignancy were found in gynecological sites and 33% in GI tract.[28–30] A comparison of various epidemiological factors related to dual primary cases in recent studies conducted has been presented in ►Table 3.
| Studies | Study period (in y) | Number of cases | Male | Female | No. of synchronous cases | No. of metachronous cases | Major primary malignancies (%) | Major second malignancies (%) | Mean time interval between metachronous cases (in y) | Median/Mean* age at primary malignancy (in y) |
|---|---|---|---|---|---|---|---|---|---|---|
| Suzuki et al [19] | 26 | 108 | 86 | 22 | 18 | 90 | H&N (61.0%) | GI (49) | 5 | 63* |
| Cheng et al [20] | 10 | 129 | 58 | 71 | 43 | 86 | GI cases only | Breast (7.7%), gynecologic (31.0%), GU (7.7%) | 8.1 | 60* |
| Irimie et al[5] | 3.4 | 63 | 29 | 34 | 22 | 41 | Breast (14.3%), gynecologic (19.0%), prostate (6.3%) | Breast (20%), gynecologic (9.5%), GI (16.5%) | 2.9 | 53* |
| Hulikal et al[6] | 5 | 38 | 17 | 21 | 13 | 25 | Breast (23.0%), H&N (55.0%), gynecologic (7.8%) | GI (18.4%), H&N (44.0%) | 6.4 | 51 |
| Bagri et al[12] | 4 | 41 | 16 | 25 | 8 | 33 | Breast (17.0%), H&N (34.0%), gynecologic (21.9%) | Breast (21.9%), GI (21.9%), H & N (12.0%) | 5.08 | 48 |
| Amer[4] | 8 | 322 | 147 | 175 | 47 | 275 | Breast (29.2), prostate (14.9%), GU (23.0%), GI (9.0%) | Breast (18.3%), GI (11.5%), prostate (6.5%) | 6.4 | 60* |
| Kim and Song[26] | 14 | 108 | 1 | 107 | 0 | 108 | Breast cases only | Thyroid (41.7%), gynecologic (15.8%), GI (14.8%) | 5 | 56 |
| Sharma et al[27] | 5 | 38 | 17 | 21 | 10 | 28 | Breast (28.9%), H&N (26.3%), gynecologic (13.0%) | Breast (23.6%), GI (10.5%), H&N (34%) | 5.5 | 52 |
| Tanjak et al[28] | 25 | 1,785 | 792 | 993 | 520 | 1,265 | Breast (23.0%), H&N (12.2%), GI (26.2%) | Breast (18.3%), H&N (11.8%), GI (29.7%) | 1.5 | 60* |
| Dutta et al[29] | 11 | 41 | 24 | 17 | 19 | 22 | Breast (12.0%), H&N (38.0%), GI (13.0%) | Breast (9.7%), GI (17.0%), H&N (34.0%) | 7.4 | 55 |
| Present study | 2 | 45 | 19 | 26 | 29 | 16 | Breast (33.3%), H&N (22.2%), gynecologic (11.1%) | GI (31.1%), gynecologic (17.8%), H&N (15.6%) | 4.7 | 59 |
Abbreviations: GI, gastrointestinal; GU, genitourinary; H&N, head and neck.
* Indicate the mean age at the diagnosis of primary malignancy.
Patients with HNSCC have known for a 36% incidence of second primary malignancy over 20 years.[21] This has been attributed to field carcinogenesis related to exposure to common risk factors like tobacco chewing, smoking, and alcohol consumption.[21,31] In our study, it is also found that H&N cancers were the most common groups to develop a new primary (10 in 45 cases; 22%) after breast primary.
Most of the synchronous dual primary malignancies were diagnosed incidentally for second tumors in our study. When such tumors are incidentally detected they should not be dismissed as metastatic diseases. Any unusual site of metastasis should be thoroughly evaluated to rule out the rare possibility of a second primary.[6]
The possibility of multiple primary malignancies should always be considered during the treatment and follow-up of cancer patients, especially those having a strong family history of cancer. Due to the potential for long-term survival when treated at early stage, more aggressive treatment may be warranted by the multidisciplinary treating oncologist team.[4] There is a high chance of multiple primary occurrence when treating the primary of the H&N and breast cancer belonging to young age and early stage. While treating the patient, the oncologist should inform the patient about the chances of multiple primary occurrences and consult the oncologist if symptoms occur. The patient should be advised a genetic counseling for the same.
As per our results on the average time for a second malignancy event, we can suggest that patients and clinicians should be aware of such occurrences that could later emerge and be ready for early diagnosis and better management of the disease for improved quality of life of the patients. This is possible when follow-up appointments are scheduled at regular intervals, patients are aware of the need for follow-up and do not avoid/miss follow-up appointments, and the treatment center has state-of-the-art facilities. As the years increase for the follow-up, there is a chance to develop a second primary. If HBCR and population-based cancer registry are located in the same geographical area then cancer registry staff should regularly follow-up on dual primary cases and encourage them to visit the hospital for follow-up and to get treated in the hospital. If the hospital is nearby to the patients who were diagnosed with multiple primaries then they can get treated effectively. We also recommend that the cancer hospital/center should develop an infrastructure that provides early detection of second tumors. The data obtained from hospital cancer registries on dual malignancies may provide in-depth information about the patient so the treating oncologist may plan some preassumed investigations in view of the risk of the development of second malignancy and essential interventions may be taken for the treatment of such type of cases at the earliest.
Conclusion
More than 70% of cases of primary tumors were in the breast, H&N, gynecological, and prostate. Of the above primary, more than 60% of the second malignancy was found in the GI tract, gynecological, and H&N sites. Around two-thirds of dual tumors are synchronous. Breast cancer cases have a higher incidence of second malignancy. We need to regularly follow-up on these cases to assess the survival of the second primary.
Authors' Contributions
S.S.: Conceptualization, methodology, pathology inputs, writing the original draft.
A.K.G.: Conceptualization, clinical inputs, assistance in writing the draft.
A.S.: Conceptualization, clinical inputs, assistance in writing the draft.
K.S.C.: Data collection, data quality control, data abstraction, assistance in writing the draft.
K.A.: Data collection, data quality control, data abstraction, assistance in writing the draft.
D.C.: Conceptualization, clinical inputs, assistance in writing the draft.
T.D.: Conceptualization, clinical inputs, assistance in writing the draft.
S.T.: Conceptualization, clinical inputs, assistance in writing the draft.
P.K.: Data collection, data quality control, data abstraction, assistance in writing the draft.
P.J.: Data collection, data quality control, data abstraction, assistance in writing the draft.
A.S.: Conceptualization, pathological inputs, assistance in writing draft.
R.S.B.: Radiologist, assistance in writing the draft.
A.B.: Conceptualization, methodology, data analysis, overall supervision, writing the original draft.
A.G.: Conceptualization, clinical inputs, assistance in writing the draft, overall supervision.
J.V.D.: Conceptualization, clinical inputs, assistance in writing the draft, overall supervision.
R.A.B.: Conceptualization, supervision, technical guidance, clinical inputs, and valuable criticism on the write-up.
Acknowledgments
We acknowledge the support received from Dr. Rajesh Dikshit (Director) and Dr. Pankaj Chturvedi (Deputy Director) of Centre for Cancer Epidemiology, ACTREC, Tata Memorial Centre Mumbai, India.
Conflict of Interest
None declared.
References
- Global Cancer Observatory: Cancer Today. Lyon: International Agency for Research on Cancer; Accessed February 08, 2022 at: https://gco.iarc.fr/today
- [Google Scholar]
- Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(03):209-249.
- [Google Scholar]
- Clinical Cancer Advances 2020: Annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol. 2020;38(10):1081.
- [CrossRef] [PubMed] [Google Scholar]
- Multiple neoplasms, single primaries, and patient survival. Cancer Manag Res. 2014;6:119-134.
- [CrossRef] [PubMed] [Google Scholar]
- Multiple primary malignancies–epidemiological analysis at a single tertiary institution. J Gastrointestin Liver Dis. 2010;19(01):69-73.
- [Google Scholar]
- Second primary malignant neoplasms: a clinicopathological analysis from a cancer centre in India. Asian Pac J Cancer Prev. 2012;13(12):6087-6091.
- [CrossRef] [PubMed] [Google Scholar]
- Multiple primary malignant neoplasms: case report and a comprehensive review of the literature. Am J Clin Oncol. 2003;26(01):79-83.
- [CrossRef] [PubMed] [Google Scholar]
- Multiple primary malignant tumors: a survey of the literature and statistical study. Am J Cancer. 1932;16:1358-1414.
- [Google Scholar]
- Multiple primary malignant neoplasms. II. Tumors of different tissues or organs. Cancer. 1961;14:231-237.
- [CrossRef] [PubMed] [Google Scholar]
- New Malignancies among Cancer Survivors: SEER Cancer Registries, 1973-2000. National Cancer Institute NIH Publ. No. 05-5302. Bethesda, MD
- [Google Scholar]
- A clinical and genetic analysis of multiple primary cancer referrals to genetics services. Eur J Hum Genet. 2015;23(05):581-587.
- [CrossRef] [PubMed] [Google Scholar]
- Double primary malignancies: a clinical & pathological analysis report from a regional cancer institute in India. Iran J Cancer Prev. 2014;7(02):66-72.
- [Google Scholar]
- Multiple primary tumors over a lifetime. Oncology (Williston Park). 2019;33(07):629384.
- [Google Scholar]
- Synchronous dual malignancy: successfully treated cases. J Cancer Res Ther. 2007;3(03):153-156.
- [CrossRef] [PubMed] [Google Scholar]
- Linkage of cancer registration with cancer treatment in the predominantly rural district: a model form Sangrur district, Punjab state, India. Int J Noncommun Dis. 2018;3:56-59.
- [CrossRef] [Google Scholar]
- (2020) Cancer Incidence and Mortality in Sangrur District, Punjab State, India: 2015–2016 (Mumbai, India) Accessed April 13, 2022 at: https://tmc.gov.in/tmh/pdf/Reports/Sangrur%20Report%202015-2016.pdf
- [Google Scholar]
- International Classification of Diseases for Oncology. World Health Organization Accessed February 08, 2022 at: https://apps.who.int/iris/handle/10665/42344
- [Google Scholar]
- CanReg5 open source software for cancer registries. Lyon: International Agency for Research on Cancer; 2008. Accessed February 08, 2022 at: http://www.iacr.com.fr/index.php?option=com_content&view=article&id=9:canreg5&catid=68&Itemid=445Date
- [Google Scholar]
- Multiple primary malignancies in the head and neck: a clinical review of 121 patients. Acta Otolaryngol Suppl (547):88-92.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical analysis of multiple primary malignancies in the digestive system: a hospital-based study. World J Gastroenterol. 2005;11(27):4215-4219.
- [CrossRef] [PubMed] [Google Scholar]
- Second primary cancers after an index head and neck cancer: subsite-specific trends in the era of human papillomavirus-associated oropharyngeal cancer. J Clin Oncol. 2011;29(06):739-746.
- [CrossRef] [PubMed] [Google Scholar]
- Determinants of completion of cancer directed treatment: an experience from a rural cancer centre, Sangrur, Punjab state, India. Ecancermedicalscience. 2021;15 1313
- [CrossRef] [Google Scholar]
- Multiple primary tumours in women following breast cancer, 1973-2000. Br J Cancer. 2006;94(11):1745-1750.
- [CrossRef] [PubMed] [Google Scholar]
- Second malignant neoplasms: assessment and strategies for risk reduction. J Clin Oncol. 2012;30(30):3734-3745.
- [CrossRef] [PubMed] [Google Scholar]
- Metachronous double primary cancer after treatment of breast cancer. Cancer Res Treat. 2015;47(01):64-71.
- [CrossRef] [PubMed] [Google Scholar]
- Second primary malignancy: a retrospective analysis report from a tertiary cancer center of North India. Indian J Cancer. 2016;53(04):595-599.
- [CrossRef] [PubMed] [Google Scholar]
- Risks and cancer associations of metachronous and synchronous multiple primary cancers: a 25-year retrospective study. BMC Cancer. 2021;21(01):1045.
- [CrossRef] [PubMed] [Google Scholar]
- Double primary – the pattern of care, and epidemiology: experience from a tertiary cancer care center. Asian J Pharm Clin Res. 2022;15(09):80-83.
- [CrossRef] [Google Scholar]
- Risk of second primary malignancies in women with breast cancer: results from the European Prospective Investigation into Cancer and Nutrition (EPIC) Int J Cancer. 2015;137(04):940-948.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomic sites at elevated risk of second primary cancer after an index head and neck cancer. Cancer Causes Control. 2011;22(05):671-679.
- [CrossRef] [PubMed] [Google Scholar]






